Human Medical Experience Provides Paradigms Relevant to Captive Breeding of Endangered Wildlife: Rationale for Prevention and Therapy of Hemolytic and Iron Overload Propensities in Browser Rhinoceroses, Tapirs, and Other Susceptible Species
Donald E. Paglia, MD
Hematology Research Laboratory, Department of Pathology and Laboratory Medicine, School of Medicine, UCLA, Los Angeles, CA, USA
Abstract
Throughout history, advances in human medicine have relied heavily on experimental studies of animals. Conversely, insights into maladies affecting a wide range of animal species may be gleaned from the established pathophysiology of clinically similar syndromes in humans. Here examples are presented that demonstrate the value of this approach as well as potential hazards in its disregard. In the 1980s and 1990s, lethal hemolytic anemia often occurred among captive African black rhinoceroses (Diceros bicornis) following exposure to certain drugs or chemicals. These episodes resembled common disorders in humans caused by impaired ability to neutralize ambient oxidants. Comparative studies revealed metabolic characteristics of rhinoceros red blood cells (RBCs) that deviated radically from all other mammals, including a dearth (2–5%) of the essential metabolic fuel, adenosine triphosphate (ATP). Strategies to preserve and enhance RBC ATP, including avoidance of oxidant stresses and introduction of high-phosphate diets, were derived from human experiences and applied to rhinoceroses with consequent reductions in hemolytic episodes. Induction of hyperphosphatemia was additionally found to be an effective therapeutic intervention capable of interdicting active hemolysis. Iron storage disease (ISD) (aka iron overload disorder, IOD) is a progressive, clinically silent, multisystem disorder of high morbidity and mortality acquired by all browser rhinoceroses, tapirs, and many other species of exotic wildlife when they are displaced from the natural environments in which they evolved. Recent demise of the captive breeding program for Sumatran rhinoceroses (Dicerorhinus sumatrensis) can be directly attributed to ISD. Extensive experience with hereditary and acquired ISD in humans provides strategies for prevention and therapy, but few institutions have adopted them despite their demonstrable effectiveness. Unlike their human analogs, these conditions in affected animals are not caused by aberrant genetic mutations but instead represent species-wide characteristics with important evolutionary implications.
Introduction
Animals have been studied since antiquity for the benefit of mankind. Aristotle performed experiments with living animals, and fundamentals of anatomy, physiology, and pathology established by Galen during the second century were based on his extensive animal dissections and observations. In more recent times, efficacy and safety of everything from cosmetics to drugs to surgical procedures have crucially relied on animal testing, so it seems entirely appropriate that lessons learned from human studies should contribute equitably back to the health and welfare of animals. Notable current and historical examples are considered here, of a hemolytic syndrome affecting captive African black rhinoceroses (Diceros bicornis) and iron storage disease (ISD) or iron overload disorder (IOD) affecting all black and Sumatran (Dicerorhinus sumatrensis) rhinoceroses so far appropriately studied in captivity.
Acute Episodic Hemolytic Anemia
Three decades ago, acute hemolytic anemia was the leading cause of death among captive black rhinoceroses, prompting intensive investigations into potential etiologies. Common causes of premature hemolysis, such as hemoglobin abnormalities and autoimmunity, were eliminated, but collaborative studies at the UCLA Hematology Research Laboratory revealed extraordinary disparities in biochemical and enzymatic characteristics between red blood cells (RBC) of all four rhinoceros species and other known mammals.32 These differences included significantly impaired capacities to neutralize reactive oxygen species (ROS) generated by ambient physiological and pathological processes.13,22-25,34 The discovery of minimal quantities (2–5%) of the essential high-energy compound, adenosine triphosphate (ATP) in rhinoceros RBCs, remains virtually unprecedented in vertebrate hematology.22-25 As the obligate fuel for cation pumping, ATP is essential for maintaining sodium/potassium gradients across cell membranes. This minimal reserve was viewed as the potential Achilles heel for erythrocyte viability, since failure of the cation pump allows influx of water, RBC expansion, and eventual lysis. Indeed, insufficient ATP is classically (but arguably) viewed by many as the terminal event in limiting normal RBC life spans and in inducing premature hemolysis in hereditary defects of erythrocyte metabolism.
The clinical phenotype of hemolytic anemia in black rhinoceroses is virtually identical to an analogous, highly common disorder in humans, glucose-6-phosphate dehydrogenase (G-6-PD) deficiency.13 Based on extensive experience with such patients, preventive strategies for rhinoceroses are proposed, including avoidance of drugs, chemicals, and foodstuffs and other conditions (such as acidosis and hypophosphatemia) that are known to initiate hemolysis in sensitive subjects.14,15 High-phosphate diets were additionally recommended because RBC ATP concentrations are well known to correlate closely with serum phosphate levels in humans. ATP concentrations also increase rapidly in rhinoceros RBCs incubated in phosphate media, with consequent decreases in their sensitivity to oxidant challenge.13 As a preventive measure, phosphate supplementation has been credited with virtual disappearance of acute hemolytic anemia over the past two decades and has been adopted by many (but not all) rhino-holding institutions. Nonetheless, another recent episode of acute hemolysis after drug administration to a black rhinoceros with marginal hypophosphatemia serves as a potent reminder that apparent success can breed complacency.
Data are presented supporting the value of dietary phosphate supplementation in black rhinos as a routine preventive measure, as well as the potential use of parenteral phosphate infusions to interdict active hemolytic crises. In 1992–1993, three clinical cases provided opportunities to extend theoretical preventive strategies into active therapeutics. Blood specimens from three female black rhinos (approximately 20, 30, and 40 years in age) were referred to the UCLA Hematology Research Laboratory from two institutions to help evaluate conditions that included severe pododermatitis, mucocutaneous ulcerations, and multiple geriatric problems. All were overtly or marginally hypophosphatemic.
One rhinoceros was maintained on dietary phosphate supplementation and sampled serially over a 3-month period to follow hematologic indices and blood chemistries. Red cell adenine nucleotide (ATP+ADP+AMP) concentrations, measured by four different assay techniques, rose progressively as hypophosphatemia was corrected, eventually reaching levels four- to fivefold higher than control means. The second rhinoceros began to hemolyze, losing two-thirds of its circulating red cells over a 3-week period. Multiple intravenous infusions of sodium phosphates were accompanied by tripling of total red cell adenine nucleotides and cessation of hemolysis. The third rhinoceros suddenly hemolyzed with gross hematuria and rapid loss of two-thirds of its circulating red cell mass. The animal was immobilized at frequent intervals to allow multiple infusions of parenteral phosphate. These were associated with rapid elevations in red cell ATP, cessation of hemolysis, erythroid regeneration, and gradual return of packed cell volumes from a nadir of 16% to 45–48% (Figure 1).
Figure 1
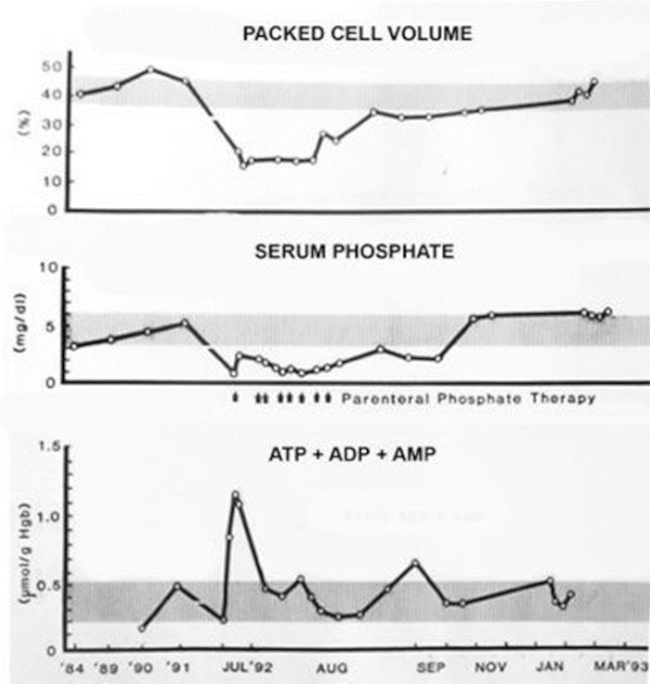
Effect of parenteral phosphate infusions on RBC packed cell volumes, serum phosphate concentrations, and total RBC adenine nucleotide concentrations in a black rhinoceros during and following an acute hemolytic episode. Shaded bars indicate ranges of normal means ± 1 SD.
These quantitative data substantiate conclusions drawn from in vitro studies regarding the importance of avoiding and/or correcting hypophosphatemia and inducing hyperphosphatemia to mitigate hemolytic tendencies in black rhinos. A recently identified mutation in the SLC28a2 gene of black (but not white) rhinoceroses likely contributes to their inherently low ATP reserves by affecting adenosine transport,7,30 a crucial nucleotide salvage pathway for mammalian erythrocytes.33 Since ATP generation is also critically dependent on glucose catabolism, avoidance of other conditions that inhibit glycolysis, such as acidosis, is an equally important preventive measure. These observations additionally support the potential value of parenteral phosphate infusions to interdict episodes of active hemolysis.
Iron Storage Disease or Iron Overload Disorder
Background
Iron storage disease (ISD) is an inexorably progressive, multisystem disorder that is typically devoid of clinical signs or symptoms until affected organ systems falter or fail. Both genetic (hereditary hemochromatosis) and acquired (transfusional) forms of ISD occur with very high frequency in humans, providing an enormous body of information regarding pathogenesis, diagnosis, treatment, and prevention that can be beneficially extended to animals.1,35
Necropsy records of virtually every African black and Sumatran rhinoceros in captivity over the past six decades cite the presence of widespread and varying degrees of hemosiderosis, the morphologic, but not necessarily pathologic, hallmark of iron deposition. In many instances, because of the high incidence of hemolytic anemia, this was erroneously interpreted as residua of previous RBC lytic episodes. Necropsy observations with iron-specific stains and quantitative tissue analyses provide unequivocal evidence of underlying pathophysiology involving iron homeostasis, resulting in clinically significant (sometimes massive) body burdens of highly toxic ferric iron.16,17,21,26-29
Due to species-wide genetic predispositions, all browser (but not grazer) rhinoceroses,17,21,27,28 tapirs,31 and many other genera of exotic wildlife3 are in jeopardy of developing ISD when displaced from natural environments where they likely evolved dependence on environmental factors or crucial dietary components to reduce iron absorption and maintain iron balance. Within weeks, excess iron loads are detectable (by serum analyte or necropsy studies) in newborns or newly captives, and these increase logarithmically, reaching tenfold elevations in as little as 3–5 years, more rapidly in Sumatran than in black rhinoceroses.16,27,28
While precise causes of ISD in some species may be uncertain, no controversy exists about its consequences. Iron in excess is invariably deleterious to biologic systems because it catalytically generates highly toxic, hydroxyl free radicals and other ROS. Rhinoceros species are particularly vulnerable to ISD due to their inherently impaired capacities to neutralize ambient oxidants that are inevitable byproducts of aerobic metabolism.13,22-26,34 The toxicity of excess iron is also a likely cause or contributor to many of the disparate disorders acquired by browser rhinoceroses under captive conditions.16,21,28,29
Diagnosis
Measurements of serum ferritin concentrations and transferrin saturation (the ratio of serum iron to total iron binding capacity, TIBC) provide the least invasive means to assess iron status. It is widely acknowledged that serum ferritin concentrations reflect total-body iron stores with an accuracy exceeded only by direct quantitative analyses of tissue samples.4-6,8 There are important caveats: ferritin is also an acute-phase reactant produced in response to inflammatory stimuli, so other clinical conditions can confound interpretation of assay results. Additionally, there can be no valid reference ranges for “normality” when an entire population is affected, as is the case for captivity-induced ISD in black and Sumatran rhinoceroses. Free-ranging rhinoceroses provide the best comparative standards.
Most rhinoceros studies have relied on species-specific ferritin assay systems developed by Smith, et al.,37 available through the Kansas State University Veterinary Diagnostic Laboratory. In separate studies, serum ferritin values measured by this assay in African black and white rhinoceroses free-ranging in their natural habitats were <100 to 200 ng/ml16,27 and <100 to ∼350 ng/ml.11 By contrast, the mean ferritin concentration of 70 adult black rhinoceroses in U.S. captivity was 7,160 ng/ml, with individual values ranging >10,000 to >100,000 ng/ml.27 Such extreme elevations are far in excess of known synthetic rates for apoferritin, thereby providing direct evidence of organ damage sufficient to release intracellular ferritin. Also by contrast, specimens from 14 captive Sumatran rhinoceroses averaged >850 ng/ml, with individual values ranging as high as 2,000–4,000 ng/ml and transferrin saturations of 90–100%, clearly indicative of significant iron overloads developing in captivity.16,27
Recently, alternative systems for measuring ferritin and assessing ISD status have been proposed,36,39 but these have not yet been validated by direct comparisons or necropsy studies. Ferritin concentrations alone may not be diagnostic of ISD, but they provide an invaluable metric of total-body iron stores when properly interpreted in context with other data, such as transferrin saturation and the ultimate determinant, histopathology with iron-specific stains.
Transferrin saturation, the amount of iron bound to the plasma transport-protein transferrin, provides a simple, qualitatively reliable supplement or alternative if ferritin assays are equivocal or unavailable. Transferrin saturation correlates well with ferritin concentrations, with quantitative tissue analyses, and with histopathology using ferric-specific stains such as Prussian blue.16,21,27 Transferrin saturation in most vertebrates is ∼35%. Values >65–70% are considered threshold for onset of overt multisystem organ pathology.1,35 U.S. captive Sumatrans measure 90–100%, clearly indicating iron in sufficient excess to overwhelm carrying capacity of protective proteins.16,21,27,28
Prevention and Therapy
Experiences over the past century with literally millions of humans with hereditary and acquired forms of ISD provide a strong foundation for extrapolation to animals. Patients with hereditary hemochromatosis typically remain asymptomatic until their fourth or fifth decade of life. If untreated, protean clinical signs and symptoms then begin to develop, reflecting endocrine, cardiac, and/or hepatic dysfunction—eventually terminating in organ failure, cirrhosis, and liver carcinomas by the age of 60. With clinical intervention, the morbidity and mortality of progressive organ dysfunction can be entirely avoided, and shortened (25–35%) life spans restored to normal, by periodically removing small aliquots of venous blood. This induces a slight anemia that signals bone marrow erythroblasts to mobilize iron stores for new RBC production, thereby averting excessive iron accumulation.
Phlebotomy protocols applicable to rhinoceroses and tapirs have been proposed18-20 and effectively applied to black rhinoceroses.9,10,12,38 It should be emphasized that this procedure is preventive (not therapeutic), and it is optimally suited for young and newly captured animals before they develop inordinate body burdens of iron. Since phlebotomy removes only ∼0.5 gram of hemoglobin iron per liter of blood, it cannot significantly reduce body burdens that reach kilogram amounts, as commonly occurs in long-term captive black and Sumatran rhinoceroses. As a therapeutic alternative, reduction of excess iron stores by pharmacologic chelation remains feasible but prohibitively expensive. Phlebotomy is contraindicated in the presence of clinical or laboratory signs of anemia or organ dysfunction, since it would only compound those problems by inducing or accentuating anemia.
Despite their demonstrable effectiveness, phlebotomy programs have not yet been widely adopted by most rhino-holding institutions, largely because of the time and expense required to train and treat animals that superficially appear to be entirely healthy until organ dysfunction becomes overt and irreversible. This makes it difficult for responsible administrators to justify commitment of resources. That reluctance might diminish if cost/effect analyses considered the following: computer programs predict sustainability of animal populations in peril by assessing factors that affect birth/death ratios. Shifts as little as ±2–6% may be capable of altering balances for certain rhinoceros populations. Based on vast experience with human ISD, preventive phlebotomy programs could increase life spans by 25–35%, perhaps equivalent to two or three more breeding cycles that could be tipping points for effective captive-breeding programs.
Evolutionary Implications
The physiologic bases of these two detrimental conditions (impaired antioxidant metabolism and propensity to overload iron) share a common denominator: both represent species-wide characteristics rather than consequences of selective genetic mutations affecting only individuals or families. This raises the obvious question: what were the evolutionary pressures that promoted these apparently deleterious characteristics?
Again, human studies may provide some clues. The equatorial belt is dominated by populations with very high incidences of hemoglobinopathies and metabolic enzyme deficiencies. These disorders have persisted despite their high morbidity and mortality, because they confer some degree of protection against the most malignant form of falciparum malaria. That selective advantage appears to be a result of lower levels of RBC ATP that characterize these conditions. Perhaps the dearth of ATP in rhinoceros RBCs provides similar protection against Babesia or other intraerythrocytic parasites? This is a hypothesis that could be tested by quantitative assays for parasite infectivity in RBCs primed to varying levels of ATP by phosphate stimulation.
Disparities between browsers’ and grazers’ tendencies to overload iron also likely relate to their evolutionary origins. Since vertebrates lack the ability to excrete iron, appropriate concentrations of this critically essential element depend on modulation of enteric uptake. In the high-oxygen atmosphere of the Oligocene epoch when browsing mammals evolved, bioavailability of iron and other essential metals was very low, favoring physiologic mechanisms for avid uptake. When grasslands became ubiquitous during the subsequent Miocene, grazing mammals emerged, and their dietary specialization required additional adaptions to prevent excessive assimilation of soil iron. In the absence of similar mechanisms to limit uptake, it seems most likely that browsers prevent iron overloading by consuming forage components that form insoluble complexes with iron allowing its enteric passage. Natural chelators such as tannins, fiber, phytates, phenolics, and other components are abundant in twigs, leaves, and bark of natural browse. L-mimosine, an amino acid with extremely high iron avidity, is concentrated in Mimosa species that are toxic to most animals but favored forage for rhinoceroses. The importance of dietary and environmental factors in iron homeostasis is emphasized by the observation that iron loading by Sumatran rhinoceroses residing in Southeast Asian sanctuaries, appears directly related to variations in their dietary and foraging tendencies.2 Without access to their natural forage, it’s not surprising that metabolic imbalances such as ISD are inevitable consequences for susceptible species.
Summary and Conclusions
The invaluable contributions that animal studies have made to the archives of human medicine may be partially reciprocated by application of the latter to the former. Studies of phenotypically similar human disorders have contributed successfully to amelioration of the hemolytic syndrome affecting black rhinoceroses in captivity. Attempts to apply similar strategies to ISD affecting multiple species of endangered wildlife have been far less effective, largely because the insidious nature of ISD allows its progression devoid of overt clinical signs or symptoms, often until it results in terminal decline. Nonetheless, experience with equivalent human disorders strongly supports repetitive phlebotomies for prevention of ISD in susceptible wildlife species with the strong probability that such programs would not only reduce morbidity and enhance quality of life, but would likely extend life spans and breeding cycles to the benefit of captive breeding programs.
Acknowledgments
The author is deeply indebted to Thomas Alvarado, DVM; Michael Barrie, DVM; and Kathryn Gamble, DVM, MS for their contributions to studies on the role of phosphate in prevention and interdiction of hemolysis. I am equally grateful to the myriad veterinarians, keepers, and institutional staffs who provided >250 blood specimens and opportunities to participate personally in performance and reviews of ∼70 necropsies of all five extant species of rhinoceroses. Studies performed in the UCLA Hematology Research Laboratory and in the field have been generously supported by grants from the National Institutes of Health (Heart, Lung and Blood and Environmental Health Sciences), International Rhino Foundation/SOS Rhino, Morris Animal Foundation, American Association of Zoo Veterinarians, LB Research and Education Foundation, and a Fulbright Senior Research Scholar Award from the Council for International Exchange of Scholars (Fulbright Foundation).
Literature Cited
1. Bothwell TH, Charlton RW, Motulsky AG. Hemochromatosis. In: Scriver CR, Beaudet AK, Sly WS, Valle D, eds. The Metabolic and Molecular Basis of Inherited Disease. 7th ed. New York, NY: McGraw-Hill; 1995:2237–2269.
2. Candra D, Radcliffe RW, Andriansyah, Khan M, Tsu I-H, Paglia DE. Browse diversity and iron loading in captive Sumatran rhinoceroses (Dicerorhinus sumatrensis): a comparison of sanctuary and zoological populations. J Zoo Wildl Med. 2012;43(3):S65–S72.
3. Clauss M, Paglia DE. Iron storage disorders in captive wild mammals: the comparative evidence. J Zoo Wildl Med. 2012;43(3):S6–S18.
4. Cook JD, Lipschitz DE, Miles LEM, Finch CA. Serum ferritin as a measure of iron stores in normal subjects. Am J Clin Nutr. 1974;27:681–687.
5. Jacobs A, Worwood M. Ferritin in serum. Clinical and biochemical implications. N Engl J Med. 1975;292:951–956.
6. Jacobs A, Millar F, Worwood M, Beamish MR, Wardrop CA. Ferritin in the serum of normal subjects and patients with iron deficiency and iron overload. Brit Med J. 1972;4:206–208.
7. Linzmeier R, Thompson R, LaMere S, Lee P, Paglia DE, Nemeth E, Ganz T. Regulation of iron balance in rhinoceroses. Proc Am Assoc Zoo Vet. 2013:36–37.
8. Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of ferritin as an index of iron stores. N Engl J Med. 1974;290:1213–1216.
9. Losey R. Large volume phlebotomy as a practical management tool for iron overload disorder. Proc 8th Biennial IRKA Workshop. 2013.
10. Losey R. Holistic approaches to combating iron overload disorder in black rhinoceros. Proc 9th Biennial IRKA Workshop. 2015.
11. Miller M, Chavey PS, Hofmeyr J, Mathebula N, Doering A, Buss P, Olea-Popelka F. Evaluation of serum ferritin and serum iron in free-ranging black rhinoceros (Diceros bicornis) as a tool to understand factors affecting iron-overload disorder. J Zoo Wildl Med. 2016;47(3):820–826.
12. Mylniczenko ND, Sullivan KE, Corcoran ME, Fleming GJ, Valdes EV. Management strategies of iron accumulation in a captive population of black rhinoceroses (Diceros bicornis). J Zoo Wildl Med. 2012;43(3):S83–S91.
13. Paglia DE. Acute episodic hemolysis in the African black rhinoceros as an analogue of human glucose-6-phosphate dehydrogenase deficiency. Am J Hematol. 1993;42:36–45.
14. Paglia DE. Rationale for phosphate supplementation in prevention and therapy of hemolytic anemia in the African black rhinoceros (Diceros bicornis). Proc Zool Soc Southern Afr. 1994a:107.
15. Paglia DE. Haemolytic anaemia in captive black rhinoceroses: potential strategies for prevention and therapy. Proc Symp Rhinos as Game Ranch Animals. 1994b:196–198.
16. Paglia DE. On the significance of hemosiderosis in captive rhinoceroses: a convergent hypothesis on the role of chronic iron overload in multiple disorders of black rhinoceroses. Proc Conf Iron Disorders Rhinos. 1999:1–60.
17. Paglia DE. Captivity acquired hemochromatosis resembling Bantu siderosis in browser, but not grazer, rhinoceroses. Blood. 2000;96:484a.
18. Paglia DE. Recommended phlebotomy protocol for prevention and therapy of chronic progressive iron toxicity in captive rhinoceroses and tapirs. Proc 3rd Biennial IRKA Workshop. 2003:1–7.
19. Paglia DE. Recommended phlebotomy guidelines for prevention and therapy of captivity-induced iron-storage disease in rhinoceroses, tapirs and other exotic wildlife. Proc Am Assoc Zoo Vet. 2004:122–127.
20. Paglia DE. Iron storage syndrome in rhinoceroses: potential role for rhino keepers in prevention and therapy. Proc 4th Biennial IRKA Workshop. 2005:1–10.
21. Paglia DE, Dennis P. Role of chronic iron overload in multiple disorders of captive black rhinoceroses (Diceros bicornis). Proc Am Assoc Zoo Vet. 1999:163–171.
22. Paglia DE, Miller RE. Increased susceptibility of black rhinoceros (Diceros bicornis) red blood cells to oxidant stress and consequent hemolysis. AAZPA Communiqué. 1992a:7.
23. Paglia DE, Miller RE. Erythrocyte ATP deficiency and acatalasemia in the black rhinoceros (Diceros bicornis) and their pathogenic roles in acute episodic hemolysis and mucocutaneous ulcerations. Proc Am Assoc Zoo Vet. 1992b:217–219.
24. Paglia DE, Miller RE. Erythrocytes of the black rhinoceros Diceros bicornis: susceptibility to oxidant-induced haemolysis. Intl Zoo Yb. 1993;32:20–27.
25. Paglia DE, Miller RE, Renner SW. Is impairment of oxidant neutralization the common denominator among diverse diseases of black rhinoceroses? Proc Am Assoc Zoo Vet. 1996:37–41.
26. Paglia DE, Radcliffe RW. Anthracycline cardiotoxicity in a black rhinoceros (Diceros bicornis): evidence for impaired antioxidant capacity compounded by iron overload. Vet Pathol. 2000;37:86–88.
27. Paglia DE, Tsu I-H. Review of laboratory and necropsy evidence for iron storage disease acquired by browser rhinoceroses. J Zoo Wildl Med. 2012;43(3):S92–S104. Erratum: J Zoo Wildl Med. 2013;44(4):1139.
28. Paglia DE, Dierenfeld ES, Tsu I-H. Pathological iron overloads acquired in captivity by browsing (but not by naturally grazing) rhinoceroses. In: Schwammer HM, Foos TJ, Fouraker M, Olson D, eds. A Research Update on Elephants and Rhinos. Vienna, Austria; 2001:217.
29. Paglia DE, Kenny DE, Dierenfeld ES, Tsu I-H. Role of excessive maternal iron in the pathogenesis of congenital leukoencephalomalacia in captive black rhinoceroses (Diceros bicornis). Am J Vet Res. 2001;62(3):343–349.
30. Paglia DE, Linzmeier R, Nemeth E, Ganz T. Genetic basis of iron storage disease (ISD) in captive black rhinoceroses (Diceros bicornis). Proc 15th Int Elephant Rhino Conserv Res Symp. 2016:42–43.
31. Paglia DE, Miller CL, Foerster SH, Wynne JE, Tsu I-H, Kenny DE. Evidence for acquired iron overload in captive tapirs (Tapirus spp.). Proc Am Assoc Zoo Vet. 2000:124–126.
32. Paglia DE, Valentine WN, Miller RE, Nakatani M, Brockway RA. Acute intravascular hemolysis in the black rhinoceros: erythrocyte enzymes and metabolic intermediates. Am J Vet Res. 1986;47:1321–1325.
33. Paglia DE, Valentine WN, Nakatani M, Brockway RA. Mechanisms of adenosine 5’-monophosphate catabolism in human erythrocytes. Blood. 1986;67:988–992.
34. Paglia DE, Weber B, Baumgarten I, Harley EH. Radiometric assessment of hexose monophosphate shunt capacity in erythrocytes of rhinoceroses. Am J Vet Res. 2001;62(7):1113–1117.
35. Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology. 2010;139(2):393–408.
36. Roth TL, Reinhart PR, Kroll JL. Development of a rhino ferritin specific assay for determining the association between serum ferritin concentrations and hemochromatosis in Sumatran rhinoceros (Dicerorhinus sumatrensis). Proc 15th Int Elephant Rhino Conserv Res Symp. 2016:68–69.
37. Smith JE, Chavey PS, Miller RE. Iron metabolism in captive black (Diceros bicornis) and white (Ceratotherium simum) rhinoceroses. J Zoo Wildl Med. 1995;26:525–531.
38. van Sonsbeek GR. Case report: treatment of iron storage disease in a black rhinoceros (Diceros bicornis) in western Europe. Proc 15th Int Elephant Rhino Conserv Res Symp. 2016:43–44.
39. Watanabe M, Roth TL, Bauer SJ, Lane A, Romick-Rosendale LE. Feasibility study of NMR based serum metabolomic profiling to animal health monitoring: a case study on iron storage disease in captive Sumatran rhinoceros (Dicerorhinus sumatrensis). PLoS ONE. 2016;11(5):e0156318. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0156318. (VIN editor: Original link was modified as of 11-16-20.)