Breathe Easy: Understanding and Handling Respiratory Complications Under Anesthesia
Sathya K. Chinnadurai, DVM, MS, DACZM, DACVAA, DACAW
Abstract
This lecture serves as a refresher on 1) respiratory physiology for non-domestic species, 2) monitoring of respiratory function under anesthesia, and 3) common respiratory anesthetic complications with an emphasis on pathophysiology and treatment. We discuss a systematic approach to the diagnosis and treatment of common peri-anesthetic respiratory derangements, such as hypoxemia and hypoventilation.
Introduction and Physiology Review
We first briefly review the typical mammalian respiratory systems and then discuss the variations on that theme, which are seen in diving mammals, birds, reptiles, amphibians, and fish. The most simplistic and yet most critical aspect of respiratory physiology is knowing that the respiratory system is always performing two primary tasks: 1) absorbing oxygen and 2) eliminating carbon dioxide. The efficiency of the respiratory system at performing those two tasks is directly related to the ratio of respiratory surface area to volume (SA:V) that is available for gas exchange. Variations in SA:V ratio can occur due to taxonomic differences and anesthetic complications, such as atelectasis and pulmonary shunting.
Anesthetic Monitor Review
The primary monitors for assessing respiratory function are the pulse oximeter, capnograph, and blood gas analyzer. Each of those monitors is discussed in more detail below.
The pulse oximeter: The pulse oximeter uses photoplethysmography to determine arterial hemoglobin saturation. It uses light emitting diodes and photosensors to detect pulsatile blood flow and determine a ratio of oxygenated to deoxygenated hemoglobin based on differential light absorption. Accurate performance depends on multiple factors, including good tissue perfusion, good pulse quality, thin epidermis, hemoglobin and oxyhemoglobin that absorb light at the required frequencies.1 Species difference in hemoglobin structure could affect the accuracy of the reading. In cases of anemia, a patient may have excellent hemoglobin saturation, but an overall deficiency in hemoglobin will still result in poor oxygen delivery. Other sources of error include patient movement, bright ambient light, and the fact that most devices are not calibrated to read accurately below 80%.
The capnograph: Capnography is arguably the most useful anesthetic monitor and often the most underused. Effective capnography can identify conditions that might lead to hypoxia before a patient is hypoxic. It is one of the most reliable ways of detecting cardiac arrest and airway obstruction. The capnograph can also be used for confirming endotracheal tube placement in challenging intubations, detecting disconnections and leaks and problems with soda lime and one-way valves.
The capnograph typically uses infrared spectrometry to determine partial pressure of CO2 in expired gas.1 This generates three pieces of information: 1) end-tidal CO2 partial pressure, 2) a capnograph waveform, and 3) a respiratory rate. For CO2 to be detected and read out on a capnograph, three steps have to take place:
1. Cellular metabolism has to produce CO2
2. Circulation has to bring CO2 from the periphery to the lungs
3. The lungs have to be ventilating for the CO2 to be exhaled and detected by the monitor
Abnormalities in end-tidal CO2 can occur due to derangements in any of those three parameters. In a healthy, normal animal, end-tidal CO2 provides a close approximation of arterial partial pressure of CO2, the indicator of ventilatory function and respiratory pH balance. Hypoventilation can cause increases in ETCO2 and PaCO2. Hyperventilation can cause decreases in both. There are also numerous scenarios when the discrepancy between expired and arterial CO2 may grow. These include any state of poor perfusion, which decreases cardiac output and pulmonary perfusion. With less blood delivered to the lungs, there is less CO2 exhaled, but there is no change in CO2 production.
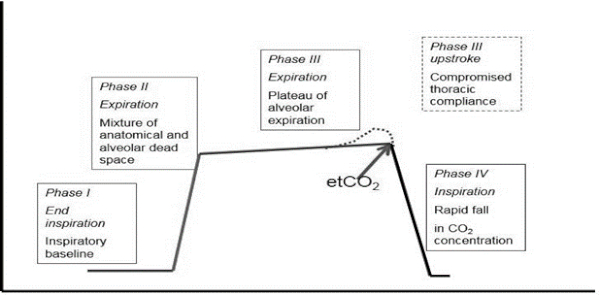
Many practitioners use their capnograph simply for one value: end-tidal CO2. That single number can be used as an effective indicator of ventilatory function, but the capnograph waveform provides many more valuable pieces of information about equipment and animal function. It can track changes in not only ventilation, but cardiac output and metabolic rate. The four phases of the capnogram that should be interpreted are pictured in Figure 1.
Figure 1. Phases of the capnogram
Blood Gas Interpretation
What can a blood gas tell you that you did not already know? Both pulse oximetry and capnography provide useful, non-invasive, second-to-second assessments of ventilation and oxygenation. But both methodologies can have some drawbacks that compromise their accuracy. Direct blood gas analysis can identify abnormalities in species for which non-invasive methodology is not validated. A capnograph can provide a non-invasive assessment of expired CO2, which should be similar to arterial CO2. Without blood gas analysis, it is possible that an increase in physiologic dead space (causes listed below) could compromise the accuracy of the capnograph. In species capable of pulmonary shunting, such as reptiles, the expired CO2 may not represent the arterial CO2 at all.
Basic interpretation should be a straightforward, step-by-step process taking 30–60 seconds. While there are more advanced ways of interpreting acid-base changes in human and veterinary critical care, they are not covered here.
Six-Step Interpretation
1. What is the pH? Normal pH for most mammals is 7.35–7.45. Is the patient’s pH low (acidemia) or high (alkalemia)?
2. What is the PO2 (arterial sample)? Normal arterial PO2 is 100 mm Hg, while breathing room air. Is the patient hypoxemic? Is the patient’s oxygen tension appropriate for the fraction of oxygen that it is breathing?
3. What is the PCO2? Normal PCO2 is 35–45 mm Hg. PCO2 represents the respiratory component of the acid-base derangement. Changes in PCO2 result in respiratory acidosis and alkalosis.
4. What is the metabolic component (HCO3 and base excess)? Are the changes appropriate for the changes in PCO2 or is there a metabolic acidosis or alkalosis?
5. What is the level of compensation? Derangements of either respiratory or metabolic acid-base balance often result in compensatory change from the other system (i.e., metabolic acidosis often results in a compensatory respiratory alkalosis [hyperventilation]).
6. Are there abnormalities in electrolytes or lactate?
An in-depth discussion of each of the six steps above is provided in the following text.
1. pH: pH only gives us the direction and extent of the derangement, but it does not tell us the source of the problem. It does help narrow down the differentials for the primary problem and the list of actions that need to be done to correct the problem. Carnivores tend to have slightly more acidic pH, while herbivores and omnivores with high carbohydrate diets tend to have more alkaline blood pH.
2. PaO2: Normal PaO2 (arterial partial pressure of oxygen) is 100 mm Hg when breathing room air and 400–500 mm Hg when breathing 100% oxygen. Hypoxemia is defined as a PaO2 <80 mm Hg. Calculating an alveolar-to-arterial oxygen gradient (A-a gradient) provides useful information about the cause of the hypoxemia. In addition, calculating a PaO2/FiO2 (partial pressure to fraction of inspired oxygen) ratio provides a very easy means of assessing pulmonary function. The A-a gradient is most accurate when the patient is breathing room air (21% oxygen), while the PaO2/FiO2 ratio can be done with any FiO2.3
A measured hypoxemia is typically the result of one of five problems:
i. Hypoventilation: The patient is not breathing frequently or deeply enough. In cases of hypoventilation, the PaO2 is low, but there is a normal A-a gradient.
ii. Low FiO2: The inspired percentage of oxygen is too low. This is rare, as most anesthetized animals are breathing an enriched oxygen mixture. The animal is hypoxemic with a normal P/F ratio.
iii. Ventilation/perfusion mismatching: This is common and is likely the main source of hypoxemia in anesthetized large animals. It is often associated with atelectasis and can be exacerbated by poor cardiac output and poor pulmonary perfusion.
iv. Diffusion impairment: Rare, is not discussed here.
v. Anatomic right to left shunt: Rare, is not discussed here.
3. PCO2: Carbon dioxide tension quantifies the balance between cellular metabolism and alveolar ventilation. Hypercapnia typically results from a decrease in ventilation, but it can be a result of increased metabolism (exertion). Hypocapnia could be from hyperventilation or decreased metabolic activity. PCO2 can also be compared to end-tidal CO2 to determine if there is an increase in physiologic dead space. End-tidal CO2 should slightly underestimate arterial CO2 by 5 mm Hg. An increase in this difference indicates that there are areas of lung that are ventilated, but not perfused. This occurs with pulmonary thromboembolism and decreased pulmonary perfusion secondary to decreased cardiac output.
4. Bicarbonate and base excess: Both of these calculated parameters provide information about metabolic alkalosis or acidosis. These are indirect measures, as both are derived from the measured CO2 on the blood gas (formulas given above). A typical reference range for HCO3 is 19–24 mEq/L. To account for the effect of CO2 on HCO3 calculation, base excess (BE) can be used. Base excess determines the amount of bicarbonate that needs to be added to blood to bring the pH back to 7.4, when PCO2 is set at 40. Essentially, base excess factors in the effect of body buffer systems and factors out the effect of CO2 on bicarbonate to determine the metabolic contribution to acid-base balance. Reference interval for bicarbonate is -4 to 4 mEq/L. While not a perfect system, BE provides a rapid way of determining the metabolic disturbance. If BE is high, there is a metabolic alkalosis, and if it is low, there is a metabolic acidosis, regardless of the respiratory disturbance.
5. Compensation: Before evaluating compensation, look back at the pH. If the pH is low, the primary process is an acidosis and the compensatory process (if present) is an alkalosis. Compensation rarely brings the patient back to a normal pH and never overcompensates. A primary chronic respiratory acidosis (hypoventilation) will lead to a compensatory metabolic alkalosis, but pH will not return to normal and will not become alkalotic. Methods of compensation include chemical buffers (few seconds), respiratory (few minutes) and metabolic compensation (few days).3
6. Lactate and electrolytes: Blood lactate is a by-product and indicator of anaerobic metabolism. Increases in blood lactate typically accompany decreases in tissue perfusion. This could include ischemic muscle from a positional or exertional myopathy in which metabolic oxygen demand has outstripped the available oxygen delivery. Focal ischemia (strangulated intestine, compromised blood flow to a limb after trauma) can also increase lactate production, and in some cases the hyperlactatemia will only be seen after perfusion is reestablished. Electrolyte interpretation is similar to routine chemistry interpretation.
This step-by-step process should lead to an assessment of oxygenation and acid-base disturbance. Acid-base disturbances are described by the pH change (acidosis or alkalosis) and the source of the disturbance (metabolic or respiratory). In many cases, there is a mixed metabolic and respiratory disturbance. A few causes of the four main acid-base disturbances in animals are listed:
Metabolic acidosis: Gastrointestinal bicarbonate loss (diarrhea), renal bicarbonate loss, lactic acidosis secondary to hypoperfusion
Metabolic alkalosis: Pyloric outflow obstruction, excessive exogenous bicarbonate therapy
Respiratory acidosis: Hypoventilation due to anesthesia, muscle relaxation, central nervous system (especially medullary or cervical) disease, airway obstruction, excessive dead space ventilation or hyperthermia
Respiratory alkalosis: Hyperventilation due to hypoxemia, pain, anxiety, inappropriate ventilator settings
Respiratory Complications Under Anesthesia
Hypoxia: In terrestrial mammals, hypoxemia is defined as an arterial partial pressure of oxygen <60 mm Hg or an SpO2 of <90%. Hypoxia occurs for one of five reasons:
1. True hypoventilation: Either the patient is not breathing enough, or it has an obstruction keeping it from breathing. In these cases, the patient has a high PaCO2 and a low PaO2.
2. Ventilation/perfusion mismatch:
a. Atelectasis
b. Dead space ventilation
3. Anatomic right-left shunt; uncommon, unless you are a reptile and are built for this
4. Low inspired FiO2 (the animal is breathing a low oxygen mixture); this is rare under anesthesia
5. Diffusion impairment; very rare
Of the above choices, 1 and 2 are, by far, the most common. Hypoventilation can be ruled in or out only with a capnograph or blood gas to determine CO2. If the ETCO2 is high and the SpO2 is low, breathe more frequently. If the ETCO2 is low or normal and the SpO2 is low, there is most likely a ventilation-perfusion mismatch, such as atelectasis. In this case, fewer, deeper, and larger breaths will help, but only increasing rate will not.
Tachypnea: Again, we often assume that if a patient’s respiratory rate increases, that it is light. In truth, there are likely four reasons why a patient is tachypneic under anesthesia:
1. It is hypercapneic
2. It is hypoxemic
3. It is light and responding to noxious stimulus
4. It is hot and trying to lose heat
Either CO2 is too high, oxygen is too low, the patient is light/stressed/painful (i.e., inadequately anesthetized), or it is too hot. Instinctually turning up the gas only addresses one of these options. When noticing any change in respiratory rate and character, do a spot check. What is the ETCO2, the SpO2, body temperature, and depth of anesthesia? If something does not make sense, run a blood gas analysis and make sure your equipment is working.
In most recumbent animals under anesthesia, there is a degree of dependent atelectasis and hypostatic vascular congestion, combined with some preferential blood flow to gravitationally dependent areas of lung. This combination of issues is what can lead to profound ventilation/perfusion mismatching under anesthesia, as the dependent areas of lung are underinflated or collapsed, but receiving the majority of the blood flow, making gas exchange very inefficient.
Atelectasis: Under anesthesia, especially with 100% oxygen, there is a significant degree of regional dependent atelectasis in almost any mammal. This is usually regional alveolar collapse, not larger lung or lobar collapse. Oxygen (100%) exacerbates this collapse, because oxygen is freely diffusible into the blood, while room air (mostly nitrogen) is not. In an animal with a lung full of 100% oxygen, even a brief apnea will result in passive movement of oxygen out of the alveolar space, into the capillary blood. Once all that oxygen has gone from the alveolus to the blood, the alveolus can collapse, causing absorption atelectasis. Under anesthesia, the only way to reliably prevent this is using positive pressure ventilation with some peak end expiratory pressure (PEEP). In the same animal breathing room air (78% nitrogen, 21% oxygen) during the same apneic period, only the 21% oxygen would absorb into the blood, leaving the 78% nitrogen skeleton to hold the alveolus open. This is why, in people (and in horses at some institutions), inhalant anesthetics are given in 40% oxygen/60% medical air, and not given in 100% oxygen.
Regarding hypostatic congestion: Under anesthesia, the vasodilation and poor peripheral blood flow that can occur, can result in interstitial (less likely within the alveolus) fluid accumulation and blood pooling. This can actually be a huge problem when an anesthetized large animal is flipped from one lateral recumbency to the other under anesthesia. Depending on the anesthetics used, the animal may not have intact vasomotor tone or sufficient baroreceptor response to redirect blood flow quickly, and the change in body position can cause profound drops in cardiac output due to poor venous return to the heart (from the areas of pooling blood). In addition, the atelectatic, previously down lung may not have time to adequately reinflate before the other lung starts to become atelectatic, resulting in worsening hypoxia.
Mechanical Ventilation
In many cases, the most effective way to combat atelectasis and hypoventilation is to use mechanical ventilation. We conclude by briefly outlining the types of mechanical ventilators available and their use.
Literature Cited
1. Dorsch JA, Dorsch SE. Understanding Anesthesia Equipment. 5th ed. Baltimore, MD: Lippincott Williams and Wilkins; 2008.
2. Hopper K, Rezende M, Haskins SC. Assessment of the effect of dilution of blood samples with sodium heparin on blood gas, electrolyte, and lactate measurements in dogs. Am J Vet Res. 2005;66(4):656–660.
3. Keller KA, Innis CJ, Tlusty MF, Kennedy A, Bean S, Cavin J, Merigo C. Metabolic and respiratory derangements associated with death in cold-stunned Kemp’s ridley turtles (Lepidochelys kempii): 32 cases (2005–2009). J Am Vet Med Assoc. 2012;240:317–323.
4. Martin L. All You Need to Know to Interpret Arterial Blood Gases. 2nd ed. Baltimore, MD: Lippincott Williams and Wilkins; 1999.
5. Magnusson L, Spahn DR. New concepts of atelectasis during general anesthesia. Br J Anaesth. 2003;91(1):61–72.