Cross-Border Multi-Agency Veterinary Response for a Free-ranging Chronically Ill Juvenile Southern Resident Killer Whale (Orcinus orca)
Abstract
Background
Southern resident killer whales (SRKWs) are a small population of endangered salmon-eating killer whales numbering 74 individuals as of October 2018. This population resides on the west coast of North America (USA and Canada). In May and September 2017, aerial photogrammetry measurements1 made by NOAA Fisheries and SR3 documented declining condition in a 3-year-old female from this population, J50 (a.k.a. Scarlet), who had been measured to be small for her age since birth. When observed again in June 2018, scientists from NOAA Fisheries and the Center for Whale Research noted that she was now noticeably emaciated with prominent loss of nuchal fat. Biologists also noted a fetid smell to her breath on at least one approach. Follow-up photogrammetry confirmed a considerable loss in body condition compared to the previous year. Through consultations with veterinarians and experts, NOAA Fisheries in the U.S. and Department of Fisheries and Oceans (DFO) in Canada worked closely with many partners and authorized an emergency response, which resulted in the first-ever attempt to provide veterinary care for a free-ranging SRKW (Figure 1).
| Figure 1. Intervention timeline | 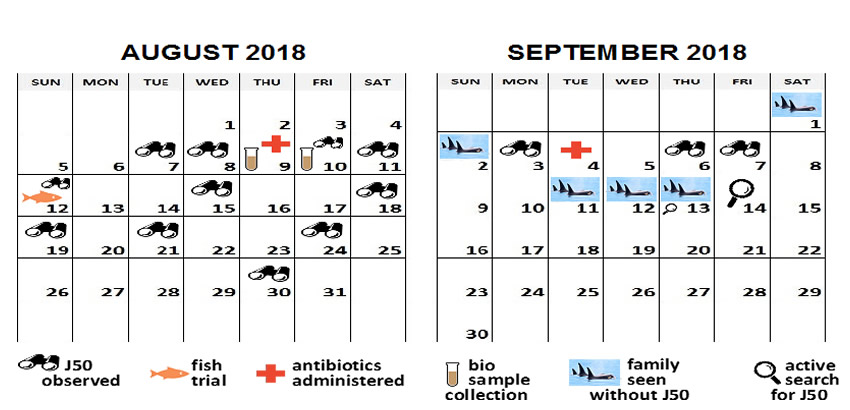
|
|
| |
Initial discussions about potential veterinary intervention for live-stranded or sick SRKWs occurred at killer whale health meetings in March 2016 and March 2017, and thereafter a subgroup of biologists and veterinarians continued to gather information and prepare for possible interventions. This early planning set the stage for convening experts to conduct a health assessment, deliberating the merits of intervening, and facilitated an intervention attempt for J50.
Diagnostic Samples
Diagnostic information was limited to observations, using both boat-based visual assessments and quantitative aerial photogrammetry as well as analysis of blow and fecal samples.
Observations by boat revealed profound emaciation with weakness (reduced stamina), intermittent separations of up to 1 mile from the pod, intermittent swimming posture with her head held higher out of the water (consistent with decreased blubber mass and therefore, buoyancy), and one occasion of fetid exhaled breath. She was not observed voiding normal feces (brown plume) during drone-based photogrammetry surveys, even though her family members were. When she had been observed with other actively foraging pod members, J50 did not appear to be engaged in pursuing prey or prey-sharing, a behavior regularly seen in resident killer whales.2 When faced with strong currents or when family members surged ahead foraging at higher speeds, J50 was unable to maintain pace with the pod. Despite often lagging behind, she consistently traveled with her mother, J16 and the J16 matriline group until September 1 and 2 when the J16 matriline group was sighted without her (Figure 1). She was re-sighted again with the J16 matriline on September 3rd. While J50 may have caught up with her pod after these events, it is likely that the J16s sought out J50 as she started lagging behind the group.
On July 21, 2018, blow samples were collected from a boat platform using an 18’ carbon fiber pole attached to a Petri dish lined with sampling mesh. They yielded small quantities of DNA. Pan-bacterial sequencing did not identify a dominant bacterial organism, while pan-fungal PCR reactions and sequencing detected a dominant organism best matched to the fungal genus, Exophiala (Ascomycetes), which is widely present in the environment (especially wood and soil) but also a known opportunistic pathogen of humans and fish where it can infect both the skin and the respiratory system.3 A second sequence matched to Cladosporium, a fungal genus that was frequently detected by culture in SRKW breath.4 A second blow sample collected on August 20, 2018 failed to detect either fungus or a dominant bacterial organism.
Photogrammetry measurements from drone-derived aerial images1 collected on August 1, 2018 revealed that J50 was ∼351 cm long, and was subsequently estimated by veterinary assessment to be ∼20% underweight (Figure 2). Extrapolating from length to body weight ratios from killer whales housed at the SeaWorld parks, and accounting for the weight loss, her body mass was estimated at 608 kg. This estimated body weight was used to calculate drug doses. Fluid maintenance requirements were estimated from the average total daily food intake of a 321 cm long female killer whale at SeaWorld. J50’s fluid and caloric requirements were estimated to be 50% higher to account for her activity budget and decreased buoyancy. The attending veterinarians deemed meeting J50’s fluid requirements, at least during the initial response, more critical than meeting her caloric requirements. While the exact correction factor for wild whales is not known and presents an opportunity to improve an intervention, there was a general agreement between caloric requirement calculations based on daily prey energy requirements data for SRKWs5,6 and extrapolations from diets of SeaWorld-housed killer whales.
| Figure 2. Aerial images of J50 showing progressive emaciation from 2017 to 2018 and between August 1 and September 3, 2018 | 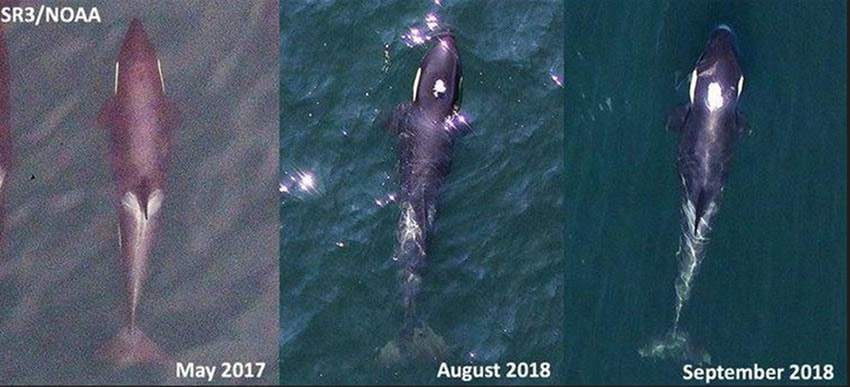
|
|
| |
Two fecal samples were collected off the water from behind the J16 matriline group using scat detection dogs on August 12 and August 18, 2018. After the analyses were completed (results below), these samples were identified using DNA fingerprinting to have been from J50's dam, J16, and another whale J27, respectively (Kim Parsons, NOAA Marine Mammal Laboratory, pers. comm.).
Fecal cytology on the August 12 sample revealed 4+ mixed bacteria and no white blood cells (WBCs). Gram stain showed 4+ Gram-negative rods (GNRs; 2 or more morphotypes), at least 1 resembled Escherichia coli, 1+ Gram-positive rods (GPRs); with spores, 2+ yeast, and no WBCs. The fecal sample collected on August 18 was more diluted than the first sample and considered less reliable. Cytology showed no WBCs, no yeast and 2+ mixed bacteria. Gram stain also failed to show WBCs or yeast and 3+ mixed bacteria, consisting of Gr+ rods with spores, Gr- rods, Gr- coccobacilli, Gr+ cocci.
Bacterial culture performed on fecal samples at two different reference laboratories yielded Vibrio parahaemolyticus from enrichment broth; small numbers of Clostridium sordellii and C. perfringens; large numbers of Photobacterium damselae; small numbers of two Escherichia coli types and small numbers of Edwardsiella tarda. A Brucella-genus specific PCR test targeting a 150 bp fragment of the gene encoding the insertion sequence IS711on the August 12 fecal sample was suspect positive but inconclusive.7
Parasitology of the August 12 sample (from J50's mother, J16) revealed Contracaecum sp. ova by direct smear, fecal floatation and sedimentation. Parasitology examination of the August 18 sample was negative for parasites and ova. The fecal sample collected on August 12, 2018 was found to be negative for saxitoxin by enzyme-linked immunosorbent assay (pers. comm. Kathi Lefebvre, NWFSC).
Fish Delivery Trial
To develop potential methods to deliver oral medications to J50, a trial using live Chinook salmon was authorized by NOAA Fisheries. On August 12, a total of 22 live Chinook were provided by the WDFW Marblemount Hatchery and transported via the Lummi Nation Enforcement Vessel. The fish transport and delivery vessel was equipped with two oxygenated saltwater totes; water temperature, oxygen levels, and Chinook health were continuously monitored throughout transport by Lummi Nation and WDFW personnel.
On the day of the Chinook deployment trial, J50, the J16 matriline group, and the larger J-Pod were continuously monitored from multiple research vessels. Criteria for the fish deployment trial to begin included J50's separation from the rest of the pod and conditions allowing for sufficient monitoring both from vessel platforms and from a drone. Trial fish deployment began when the J16s transitioned into foraging behavior and J50 fell behind the group. NOAA biologists determined sufficient separation from the group.
A fish deployment system was developed to minimize risk of J50 associating the Chinook with the delivery vessel. This system involved attaching an 8in diameter PVC pipe to the back of the vessel at an angle that allowed for the Chinook to be placed in the pipe on deck, and slide into the water at approximately 12in below the surface of the water and 2ft behind the transom of the vessel. Deploying the fish into the water in the bubble slipstream behind the vessel was intended to minimize association of the fish with the acoustic signature of the vessel.
UAV video and vessel-based monitoring were conducted throughout the trial to both assess the response of J50 to the Chinook, and to monitor for non-target animal interactions. Chinook were deployed approximately 50 m directly ahead of J50 while she was separated from the group and swimming in a predictable direction. Two research vessels trailed behind J50 during the fish deployment for collection of prey and fecal samples, as well as to collect images used for monitoring.
Once fish deployment began, only the healthiest looking and most robust fish were considered for release. A scale was collected and cataloged from each fish just prior to deployment, for future analysis should a prey scale sample or a fecal sample be collected. A total of 8 live Chinook ranging from between 6–16 lb were deployed during this attempt; however, it did not appear as though J50 responded to or consumed any of the fish based upon on-water observations and review of the UAV footage. Factors contributing to this may have included suboptimal tidal current conditions and the use of hatchery fish that had already entered a fresh water system. It is unclear whether any of the other killer whales ingested any of the fish.
Future development of this method for potential oral medication delivery to a free-swimming killer whale will require additional data on the fish detection range of killer whales and further development of monitoring methods to ensure the target animal consumes the fish.
Based on the results of the fish feeding trial, there was higher confidence in delivering antimicrobial medications using a remotely delivered dart.
Therapy
Killer whale necropsy findings show that bacterial pneumonia is often present in dead stranded killer whales (pers. comm. S. Raverty; kw health database). Despite lack of definitive laboratory evidence for a bacterial infection, J50 was suspected to have some degree of respiratory compromise by the attending veterinarians. As pneumonia is often fatal in cetaceans, the veterinarians recommended remote delivery of a long-acting antibiotic to J50, which was authorized under permit by NOAA Fisheries and DFO. Cefovecin (Convenia®) was chosen due to its demonstrated safety in other odontocetes and because it could be concentrated for delivery and provide sustained tissue levels once administered (pers. comm. M. Papich). Needle length was chosen based on past data regarding skin and blubber thickness for similar aged animals. Three grams (600 kgx5 mg/kg) of cefovecin was concentrated into 10 ml and partially administered via remote delivery August 9th and again on September 4th using a 10 ml Dan-Inject® air-pressurized dart with 2"x60 mm straight needle, which was later changed to a collared needle to reduce the possibility of the dart bouncing out of the animal before drug delivery was complete. During both darting attempts, some of the medication was observed to spray out at the dart entry site, indicating the full dose was not injected. Continued evaluation of the dart, needle, and blubber thickness and quantifying the loss of medication as the dart enters the skin and blubber layer will lead to process improvement for future interventions.
Based on the identification of Contracaecum sp. in the August 12 fecal sample, which was later identified to be from J50's mother (J16), veterinarians recommended administering ivermectin in an attempt to reduce parasite load as a secondary stressor. Initial delivery of ivermectin at 0.2 mg/kg using a 10 ml Dan-Inject® air-pressurized dart with 2"x60 mm collared needle was attempted on September 7, but the delivery failed due to missed darting attempts.
Rescue Contingency Planning
While observations and samples were collected and analyzed and medication was provided remotely, extensive discussion and planning continued weighing the benefits and risks of capture and physical health assessment, short- or long-term rehabilitation, and release. The goal of any such intervention was survival of J50 in the wild as a reproductively contributing member of the population, while avoiding harm to other members of the population. Scenarios supporting a rescue, such as separation from her pod or stranding, were identified, but additional development of response plans for future situations and identification of resources for future interventions are ongoing.
Outcome
Unfortunately, the efforts to diagnose and treat J50 were not successful. Quantitative analysis of additional aerial photogrammetry images of J50 collected on September 3, 2018 showed continued progressive decline since August 1, 2018 and extreme emaciation (Figure 2). J50 continued to lose body condition and was last sighted by the biologists and the veterinary team on September 7th during an attempt to administer ivermectin. A whale watch operator retrospectively identified J50 from photos on September 8th, but she was not sighted again and was declared dead after several days of intensive searching and multiple sightings of her family group without her.
Lessons Learned
Despite the eventual loss of J50, the intervention team learned a lot from this unprecedented effort. With the significant improvements in veterinary diagnostics and treatments, the expertise to design and conduct a more effective diagnostic and medical intervention scheme exists and should be harnessed to adapt techniques for free swimming cetaceans, particularly SRKWs. These techniques and approaches can then be used to develop a well-coordinated US-Canadian transboundary effort, such as with the previous success with A73, a lone Northern Resident killer whale calf that was rescued in Puget Sound and returned to her family group in Canada. People from myriad organizations and agencies came together and provided in-kind funding to help with the emergency response for J50: Abbotsford Animal Health Centre, Canada's Fisheries and Oceans, Center for Whale Research, King County, The Lummi Nation, National Marine Mammal Foundation, NOAA Fisheries, Orca Research Trust, SeaDoc Society (UC Davis School of Veterinary Medicine), SeaWorld, Soundwatch and the Whale Museum, SR3, University of Washington, Vancouver Aquarium, Washington Department of Fish and Wildlife, Whale Sanctuary Project and Wild Orca were all major contributors. There was intense interest in the emergency response for J50 and communications about the response, including the health and medical treatment, were critical. NOAA Fisheries provided regular updates through social media, a dedicated web page,9 and the media regarding the response activities and held public meetings to raise awareness about J50 and recovery efforts for the SRKW population.
We are still limited by our ability to remotely diagnose, and therefore treat disease, injuries or other conditions limiting recovery of critically endangered free-swimming killer whales. Despite our best efforts using her clinical history, visual assessments, behavioral assessments, and evaluation of exhaled breath and fecal samples, we never identified a cause for her sickness. Consequently, treatment attempts focused on addressing potential likely secondary factors such as bacterial pneumonia and parasitism. Better non-invasive and minimally invasive sampling and testing and better intervention and delivery tools will improve our ability to understand causes and contributing factors allowing efficient delivery of more specific medical assistance/intervention in the future. Specifically, the following pros and cons and future needs were identified:
- The 2016 and 2017 early SRKW health assessment planning meetings were of great value to this intervention attempt; because of these meetings, most interested stakeholders and relevant experts had already been identified.
- Despite not having a formal MOU for such interventions, the US and Canadian Federal Governments did an excellent job collaborating on response for an animal that was swimming on both sides of the border almost daily.
- This intervention was not planned and funding for such an operation also had not been identified prior to this event. All of the participants donated personnel time and resources. In the future, a priori contingency planning and funding for such operations would be ideal.
- Biologists routinely sample SRKWs. Many of the samples collected for research purposes also have diagnostic potential. This sample resource is currently under-used for health assessments of SRKWs.
- There is a need to create better tools for the diagnostic evaluation from non-invasive or remotely collected samples, specifically blow and fecal samples (metabolomics, proteomics, etc.). These new tools should be validated with animals with known medical histories (captive and wild) and be used to establish baselines on healthy and non-healthy wild animals. Discussions with the technical experts should include the potential to use these types of samples more broadly for health surveillance or comparisons for establishing baselines. No actual diagnosis for J50 was made and there is a need to create better tools for the diagnostic evaluation from non-invasive, minimally invasive or remotely collected samples. Research and development should focus on creating more diagnostic tools to collect more information from blow and fecal samples (metabolomics, proteomics, etc.). These new tools should be validated with animals with known medical histories (captive and wild) and be used to establish seasonal baselines on healthy and non-healthy wild animals.
- Despite the cosmopolitan distribution of killer whales, still relatively little is known about causes of morbidity and mortality in free-ranging animals. Diagnosing pathology of stranded animals, baseline sampling of healthy animals, and completion of a robust electronic medical records system for killer whales will give us a better understanding of naturally occurring disease, contributing factors, and causes of death in these animals.
- Aerial unmanned vehicles have great and unique potential for health assessment of wild SRKWs. Still images from a drone are excellent for assessing body condition in a quantitative way and having drone video or photobursts could be helpful for collecting additional valuable observations, such as appetite, food consumption during prey sharing, respiratory rates, presence/absence of defecations, interaction with the rest of the pod, dive duration and depth relative to the rest of the pod, etc. Collecting these additional data will ideally require a dedicated drone platform. We are currently limited by a small number of trained and permitted drone pilots with southern resident killer whale identification experience.
- Using suction-cup adhered D-tags, camera tags or other newer technology would provide additional information on dive frequency, duration, depth, and potentially heart rate. Tags were not used on J50 due to concerns about affecting hydrodynamics in an already compromised individual, but this technology may be useful for diagnostics in future cases.
- We did not have most of the supplies needed for this intervention, and what we did have was centrally located for diagnostics, treatment, or for a rescue. In the future, all supplies should be concentrated in one area, preferably with easy access to a dock and airstrip.
It is important to remember that the people working overtime to help J50 never lost sight of the bigger picture - the need to take the major ecosystem-level actions necessary to recover the entire SRKW population. The response to J50 was implemented in the context of the broad recovery program for the endangered SRKWs including increasing salmon, decreasing underwater noise, and reducing man-made contaminants. One of the models we've studied for successfully intervening in free-ranging wildlife is SeaDoc's sister program, the Gorilla Doctors, who have provided health care for endangered mountain gorillas in central Africa for over two decades. This example of extreme veterinary intervention has been responsible for half of the 4% growth rate achieved in these endangered animals.8 While veterinary intervention to remove snares or treat respiratory disease passed from people to gorillas was ongoing, biologists never stopped addressing other threats. Individual animal care is important for small, endangered populations, but it won't help the population without simultaneous ecosystem-level recovery efforts.
Veterinarians and biologists involved in the J50 intervention will continue to move forward to improve tools for diagnosing and treating disease in free-ranging killer whales and for responding to killer whale emergencies such as a live stranding or in the case of an oil spill. We have even created individual animal health records to more clearly follow the health of individuals over time. These efforts complement and contribute to efforts to improve the ecosystem for these animals.
Acknowledgments
In the U.S., emergency response activities were authorized under NMFS Permit No. 18786-03 (Co-Investigator letters issued to Gaydos, Haulena, Nollens, Hanson and Foster) to perform veterinary health assessments, treatment and intervention for endangered Southern Resident killer whales. In Canada permission was granted under DFO Permit No. 2018-39-PPAC-18-00030 for NOAA, DFO and expert veterinary intervention (issued to Haulena et al.). Aerial photogrammetry was performed under NFMS Permit No. 19091. We thank the US Coast Guard, other vessel and aerial observers, and the stranding network for extensive help searching for J50 after she was last sighted.
We thank the hundreds of veterinarians, biologists, whale watchers and private citizens that assisted with this effort, mostly without compensation.
Funding from NOAA Fisheries, the National Fish and Wildlife Foundation killer whale conservation program, Microsoft's AI for Earth Program, Patagonia Environmental Grants, and numerous private individuals are supporting on-going efforts to create killer whale health records.
* Presenting authors
Literature Cited
1. Durban JW, Fearnbach H, Barrett-Lennard LG, Perryman WL, Leroi DJ. 2015. Photogrammetry of killer whales using a small hexacopter launched at sea. Journal of Unmanned Vehicle Systems. 3(3):131–135.
2. Ford JK, GM Ellis. 2006. Selective foraging by fish-eating killer whales Orcinus orca in British Columbia. Marine Ecology Progress Series. 316:185–199.
3. Zeng JS, DA Sutton, AW Fothergill, MG Rinaldi, MJ Harrak, GS deHoog. 2007. Spectrum of clinically relevant Exophiala species in the United States. Journal of Clinical Microbiology. 45(11):3713–3720.
4. Raverty SA, Rhodes LD, Zabek E, Eshghi, E, Cameron, CE, Hanson MB, Schroeder JP. 2017. Respiratory microbiome of endangered southern resident killer whales and microbiota of surrounding sea surface microlayer in the Eastern North Pacific. Scientific Reports. 7:394; DOI:10.1038/s41598-017-00457-5.
5. Noren D. 2011. Estimated field metabolic rates and prey requirements of resident killer whales. Marine Mammal Science. 27(1):60–77.
6. O'Neill, SM, GM Ylitalo, JE West. 2014. Energy content of Pacific salmon as prey of northern and southern resident killer whales. Endangered Species Research. 25:265–281.
7. Wu Q, WE McFee, T Goldstein, RV Tiller, L Schwacke. 2014. Real-time PCR assays for detection of Brucella spp. and the identification of genotype ST27 in bottlenose dolphins (Tursiops truncatus). Journal of Microbiological Methods. 100:99–104.
8. Robbins MM, Gray M, Fawcett KA, Nutter FB, Uwingeli P, et al. 2011. Extreme conservation leads to recovery of the Virunga mountain gorillas. PLoS One. 6(6):e19788. doi:10.1371/journal.pone.0019788
9. www.westcoast.fisheries.noaa.gov/protected_species/marine_mammals/killer_whale/updates-j50-j35.html