M. Scherk, DABVP (Feline Practice)
Lower urinary tract disorders are common in cats. In previous decades, the focus of study has been on causes and management of crystalluria. As struvite crystalluria was successfully addressed through nutritional changes resulting in urine acidification, the frequency of calcium oxalate crystalluria increased. This encouraged emphasis on urine relative supersaturation (RSS), concentration, and a pH neutrality. Nevertheless, cats still present with characteristic lower urinary tract signs (LUTS), namely dysuria, pollakiuria, hematuria, stranguria, and periuria. The cause of approximately 65% of non-obstructive lower urinary tract disease is of unknown despite appropriate diagnostic testing. (Possible causes of LUTS are shown in Figure 1. A diagnostic approach to cats with lower urinary tract signs is shown in Figure 2.) These patients are described as having an idiopathic cystitis (IC). It is likely that this syndrome is multifactorial even within the same cat. The course of human interstitial/idiopathic, including interstitial cystitis, is known to be impacted by stress. There is evidence that there are immunological and neuroendocrine components in our feline IC patients as well.
| Figure 1 | 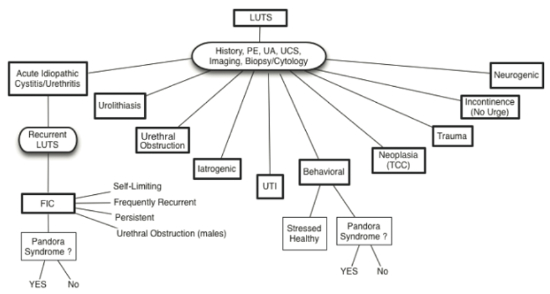
Possible causes of lower urinary tract signs in cats with or without co-morbid conditions (Pandora Syndrome) (from Chew D, Buffington CAT, FLUTH Symposium, 2014) |
|
| |
| Figure 2 | 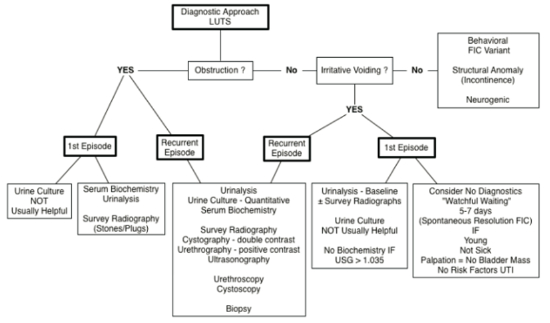
Diagnostic approach to cats with lower urinary tract signs (from Chew D, Buffington CAT, FLUTH Symposium, 2014) |
|
| |
Studying feline idiopathic cystitis (FIC) is extremely challenging not only because of its multifactorial nature, but also because clinical signs are self-limiting. In approximately 91% of cats, evidence of discomfort resolves within 7 days without treatment. Subsequent episodes are also acute in nature and occur once or twice a year. As cats get older, the frequency and severity of the flare-up decreases. A small number of cats experience chronic persistent disease lasting weeks to months.
Inflammation associated with each incident may result in functional or mechanical obstruction. The first may be caused by urethra swelling, spasm, or reflex dyssynergia, while accumulations of inflammatory debris or the formation of matrix plugs can cause mechanical obstruction. Urachal diverticulae are a possible sequelae to FIC.
What causes the inflammation in non-obstructed LUTD? Many studies have attempted to answer this question yet results have been disappointing. Infectious agents, dietary causes (mineral composition, RSS, and urine pH), neurogenic, anatomic, traumatic, neoplastic, and iatrogenic etiologies are all implicated in some individuals, but the largest category remains idiopathic in origin.
Buffington and colleagues have investigated the problem from another angle asking whether a susceptible individual might develop FIC if they are in a provocative environment. Indeed, similar to the human model of IC, he found that affected cats have structurally altered adrenals, more reactive somatosensory spinal tracts, and a larger pontine locus coeruleus (LC, the most important source of norepinephrine in the CNS) This suggests that patients with IC have increased sympathetic nervous system (SNS) activity even during periods without clinical signs. He has reviewed published epidemiologic data regarding the role of environment and its physiologic effects on risk for disease, especially in susceptible individuals. External influences include excessive body condition, decreased activity, being restricted to eliminate in a litter box, being strictly indoors, relocation of home, living with other cats, and weather changes. Stressors (internal/perceived influences) that affect different individuals to a greater or lesser degree include an impoverished environment, lack of stimulation, noise, restraint, and lack of control over his/her environment (including meals). The stress response invokes changes in immune, neurologic, and vascular status, all of which can cooperatively result in inflammation. With sufficiently severe stress, sensory input, and inflammatory mediators stimulate the hypothalamic-pituitary-adrenal axis (HPAA) and the aforementioned pontine LC - norepinephrine system. With chronic stimulation, over time normal control is lost and affected individuals overreact physiologically to threatening or disruptive situations.
| 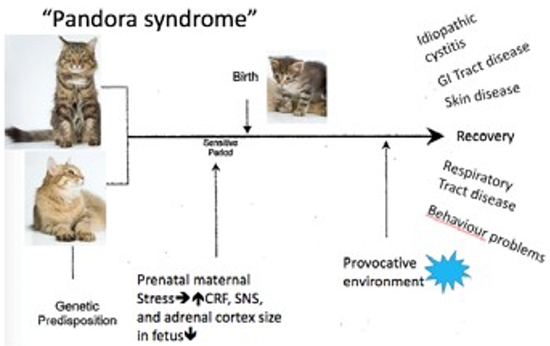
Modified from Buffington CAT, Westropp JL, Chew DJ. From FUS to Pandora syndrome: Where are we, how did we get here, and where to now? J Feline Med Surg. 2014;16(5):385–94. |
|
| |
Buffington and co-workers also identified that cats, as humans, with IC frequently have co-morbidities and has called this the Pandora Syndrome. He suggests that the bladder, rather than being the perpetrator of the LUTS, may be a victim of the systemic process associated with the sensitized central stress response system. Comorbid disorders include behavioural, endocrine, dermatological, respiratory, cardiovascular, and gastrointestinal problems. FIC does not necessarily precede the other conditions. In humans, the effects of chronic in utero stress on the health of the offspring are well documented. It may well be that genetic and similar epigenetic events contribute to the susceptibility of an individual making them at risk should they be exposed to provocative events.
Management of Cats With FIC
Evaluating the efficacy of therapies for FIC is very difficult because of the waxing-waning nature of the disorder. Stress reduction appears to be a cornerstone for managing cats afflicted with FIC. Addressing environmental needs is essential (not optional) for optimum wellbeing of the cat. Environmental needs include those relating not only to the cat’s physical surroundings (indoors or outdoors; in the home environment or at the veterinary practice) but also those affecting social interaction, including responses to human contact. Cats need to have multiple and separate locations for each resource (food, water, clean litter, toys, stable scratching surfaces, perches and resting areas). The overview of a therapeutic and management approach to a cat with LUTS is shown in Figure 3.
| Figure 3 | 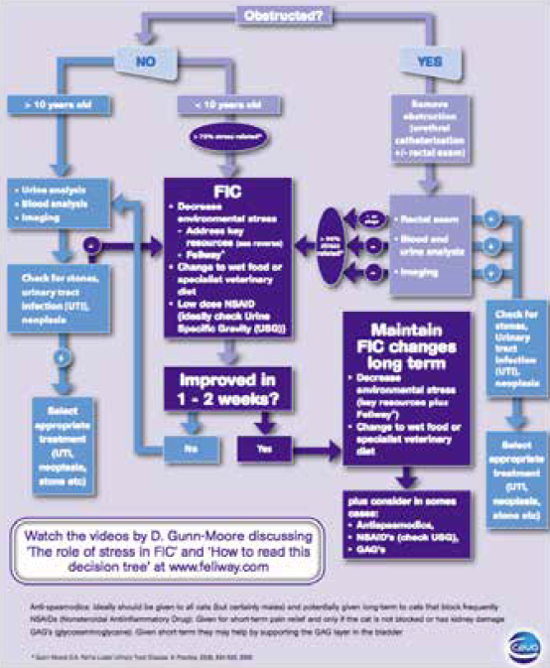
Algorithm showing a therapeutic and management approach to a cat with lower urinary tract disease. |
|
| |
It is essential that cats are able to express their natural behaviours. Cats use olfactory and chemical information to evaluate their surroundings and maximize their sense of security, comfort, and feel in control of their surroundings/environment. Depositing pheromones through cheek and paw pad marking as well as urine is key for a cat’s sense of control. In some situations, when a cat is marking with urine, it may be possible to get the cat to make a less offensive mark (from a human perspective). Cheek marking wall corners may be encouraged by using Feliway and not washing the cat’s natural oils off walls and furniture. Likewise, providing secure, stable, scratching surface placed in the location being urine marked, may result in the cat scratching and marking in that manner rather than spraying. The AAFP and ISFM Feline Environmental Needs Guidelines is an excellent resource freely available from: (jfm.sagepub.com/content/15/3/219.full.pdf+html).
Pheromone Use
Feliway™ is a synthetic analog of a feline facial pheromone that is thought to increase emotional stability. Its use in the reduction of inappropriate urination needs to be studied further. Studies done to date have shown a reduction in urine marking of less than three months duration of over 96%. In cats who had been marking for four months or longer, there was a reduction of marking in 91% of cats after 35 days of environmental treatment. A third study showed that while there was a significant reduction in all households in which Feliway™ was applied, 2/3 of the households still experienced some marking.
The product is sprayed directly on places soiled by the cat and also any prominent vertical locations in the environment. A daily application is necessary until the cat is noted to exhibit facial rubbing on the site. If the cat does not exhibit facial rubbing, then daily application to the environment should be continued for one month. Plug-in diffusers provide a constant, slow release of pheromone covering an area of 500 to 700 square feet (50–70 m2), but must not be covered, placed behind a door, or under furniture.
Diet and Drugs
Feeding a diet that produces dilute urine with a neutral pH seems to help cats have fewer recurrences of FIC or any type of lower urinary tract disease. Canned food helps to ensure that the urine is dilute, making it less concentrated (hence, less irritating) and reducing the chance that crystals can form. Having plenty of fresh water available in multiple places in a form the individual cat likes will encourage drinking. Some cats prefer drinking from a recirculating water fountain, others prefer wide bowls. Feeding a diet that has omega-3 fatty acids along with anti-oxidants may also provide beneficial anti-inflammatory effects. Finally, being consistent both in time of feeding as well as diet being fed is very important in reducing stress.
Many drugs have been used to try to reduce the reoccurrence of FIC. Amitriptyline may be helpful in some cats if it is given on an ongoing basis. It is an antidepressant and agent that stabilizes mast cells which may degranulate in some individuals with FIC. Glucosaminoglycans have also been studied and have variable, but generally poor, results. Best results appear to occur with diet, environmental, and stress management rather than drug therapy.
Summary
Lower urinary tract disorders are common in cats. Once appropriate diagnostics have ruled out direct causes, for most cases of non-obstructive LUTD, a more global approach needs to be taken, looking at and addressing the role of the cat’s external and internal environments.
References
1. Achar E, Achar RAN, Paiva TB, et al. Amitriptyline eliminates calculi through urinary tract smooth muscle relaxation. Kidney Int. 2003;64:1356–1364.
2. Achar E, Maciel TT, Collares CF, et al. Amitriptyline attenuates interstitial inflammation and ameliorates the progression of renal fibrosis. Kidney Int. 2009;75:596–604.
3. Bass M, Howard J, Gerber B, et al. Retrospective study of indications for and outcome of perineal urethrostomy in cats. J Small Anim Pract. 2005;46:227–231.
4. Buffington CAT, Chew DJ, DiBartola SP. Lower urinary tract disease in cats: Is diet still a cause? J Am Vet Med Assoc. 1994;205(11):1524–1527.
5. Buffington CAT, Chew DJ, DiBartola SP. Interstitial cystitis in cats. Vet Clin North Am Small Anim Pract. 1996;26(2):317–326.
6. Buffington CAT, Chew DJ, Woodworth BE. Feline interstitial cystitis. J Am Vet Med Assoc. 1999;215(5):682–687.
7. Buffington CAT. External and internal influences on disease risk in cats. J Am Vet Med Assoc. 2002;220(7):994–1002.
8. Buffington CAT, Westropp JL, Chew DJ, et al. Risk factors associated with clinical signs of lower urinary tract disease in indoor-housed cats. J Am Vet Med Assoc. 2006;228(5):722–725.
9. Buffington CAT, Westropp JL, Chew DJ, et al. Clinical evaluation of multi- modal environmental modification (MEMO) in the management of cats with idiopathic cystitis. J Fel Med Surg. 2006;8:241–268.
10. Buffington CAT, Westropp JL, Chew DJ. From FUS to Pandora Syndrome. J Fel Med Surg. 2014;16:385–394.
11. Calder PC. N-3 polyunsaturated fatty acids, inflammation, and inflammatory disease. Am J Clin Nutr. 2006;83:1505S–1519S.
12. Cameron E, Casey RA, Bradshaw JWS, et al. A study of the environmental and behavioural factors that may be associated with feline idiopathic cystitis. J Sm Anim Pract. 2004;45:144–147.
13. Davies M. Urinary system. In: Davidson M, Else R, Lumsden J, eds. Manual of Small Animal Clinical Pathology. Cheltenham, UK: BSAVA; 1998:287–336.
14. Defauw PAM, Van de Maele I, Duchateau L, et al. Risk factors and clinical presentation of cats with feline idiopathic cystitis. J Fel Med Surg. 2011;13:967–975.
15. Ellis SLH, Rodan I, Heath S, et al. AAFP and ISFM Feline Environmental Needs Guidelines. J Fel Med Surg. 2013;15:219–230.
16. Gerber B, Boretti FS, Kley S, et al. Evaluation of clinical signs and causes of lower urinary tract disease in European cats. J Small Anim Pract. 2005;46:571–577.
17. Gerber B, Eichenberger S, Reusch CE. Guarded long-term prognosis in male cats with urethral obstruction. J Fel Med Surg. 2008;10:16–23.
18. Gluhek T, Bartges JW, Callens A, et al. Evaluation of 3 struvite-oxalate preventive diets in healthy cats. J Vet Intern Med. 2012;26:801.
19. Gunn-Moore DA. Feline lower urinary tract disease. In Practice. 2000;22(9):534–542.
20. Gunn-Moore DA. Pathophysiology of feline lower urinary tract disease (FLUTD). UK Vet. 2001;6(5):20–26.
21. Gunn-Moore DA. Treatment of feline lower urinary tract disease (FLUTD). UK Vet. 2001;6(5):27–32.
22. Gunn-Moore DA. Investigation of feline lower urinary tract disease (FLUTD). UK Vet. 2002;7(1):49–58.
23. Gunn-Moore DA. Feline lower urinary tract disease. In: Proceedings of the ESFM Feline Congress, Stockholm 2002. J Fel Med Surg. 2003;5(2):134–138.
24. Gunn-Moore DA, Cameron ME. A pilot study using synthetic feline facial pheromone for the management of feline idiopathic cystitis. J Fel Med Surg. 2004;6:133–138.
25. Gunn-Moore DA, Shenoy CM. Oral glucosamine and the management of feline idiopathic cystitis. J Fel Med Surg. 2004;6:219–225.
26. Hostutler RA, Chew DJ, DiBartola SP. Recent concepts in feline lower urinary tract disease. Vet Clin Small Anim. 2005;35:147–170.
27. Houston D, Moore A, Favrin M, et al. Feline urethral plugs and bladder uroliths: a review of 5484 submissions 1998–2003. Can Vet J. 2003;44:974.
28. Houston DM, Rinkardt NE, Hilton J. Evaluation of the efficacy of a commercial diet in the dissolution of feline struvite bladder uroliths. Vet Ther. 2004;5:187.
29. Houston DM, Moore AEP. Canine and feline urolithiasis: examination of over 50,000 urolith submissions to the Canadian Veterinary Urolith Centre from 1998 to 2008. Can Vet J. 2009;50:1263.
30. Houston DM, Weese HE, Evason MD, et al. A diet with a struvite relative supersaturation of less than 1 is effective in dissolving struvite stones in vivo. Br J Nutr. 2011;106:S90–S92.
31. Kalkstein TS, Kruger JM, Osborne CA. Feline idiopathic lower urinary tract disease. Part I. Clinical manifestations. Comp Cont Ed Pract Vet. 1999;21(1):15–26; Part II. Potential causes. Comp Cont Ed Pract Vet. 1999:21(2):148–154; Part III. Diagnosis. Comp Cont Ed Pract Vet. 1999;21(5):387–448; Part IV. Therapeutic options. Comp Cont Ed Pract Vet. 1999;21(6):497–509.
32. Kraijer M, Fink-Gremmels J, Nickel RF. The short-term clinical efficiency of amitriptyline in the management of idiopathic feline lower urinary tract disease: a controlled clinical study. J Fel Med Surg. 2003;5:191–196.
33. Kruger JM, Osborne CA, Goyal SM, et al. Clinical evaluation of cats with lower urinary tract disease. J Am Vet Med Assoc. 1991;199:211–216.
34. Kruger JM, Conway TS, Kaneene JB, et al. Randomized controlled trial of the efficacy of short-term amitriptyline administration for treatment of acute, nonobstructive, idiopathic lower urinary tract disease in cats. J Am Vet Med Assoc. 2003;222(6):749–758.
35. Kruger JM, Lulich JP, Merrills J, et al. A year-long prospective, randomized, double-masked study of nutrition on feline idiopathic cystitis (abstr). In: Proceedings of the Annual ACVIM Forum. 2013:504.
36. Lee JA, Drobatz KJ. Characterization of the clinical characteristics, electrolytes, acid-base, and renal parameters in male cats with urethral obstruction. J Vet Emerg Crit Care. 2003;13(4):227–233.
37. Lekcharoensuk C, Osborne CA, Lulich JP. Epidemiological study of risk factors for lower urinary tract diseases in cats. J Am Vet Med Assoc. 2001;218(9):1429–1435.
38. Lulich JP, Osborne CA. Management of urocystoliths by voiding urohydropropulsion. Vet Clin North Am Small Anim Pract. 1996;26(3):629–638.
39. Lulich JP, Kruger JM, MacLeay J, et al. Struvite urolith dissolution in cats: a double-masked randomized clinical trial of two foods. J Vet Intern Med. 2011;25:747.
40. Markwell PJ, Buffington CAT, Chew DJ, et al. Clinical evaluation of commercially available urinary acidification diets in the management of idiopathic cystitis in cats. J Am Vet Med Assoc. 1999;214(3):361–365.
41. Mayer-Ronne B, Goldstein RE, Erb HN. Urinary tract infections in cats with hyperthyroidism, diabetes mellitus and chronic kidney disease. J Fel Med Surg. 2006;9(2):124–132.
42. Ortiz-Alvarado O, Miyaoka R, Kriedberg C, et al. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid in the management of hypercalciuric stone formers. Urology. 2012;79:282–286.
43. Osborne CA, Kruger JP, Lulich JP, et al. Feline matrix-crystalline urethral plugs: A unifying hypothesis of causes. J Sm Anim Pract. 1992;33:172–177.
44. Osborne CA, Lulich JP, Kruger JM, et al. Feline urethral plugs: Etiology and pathophysiology. Vet Clin North Am Small Anim Pract. 1996;26:233–253.
45. Straeter-Knowlen IM, Marks SL, Speth RC, et al. Effect of succinylcholine, diazepam, and dantrolene on the urethral pressure profile of anesthetized, healthy, sexually intact male cats. Am J Vet Res. 1994;55(12):1739–1744.
46. Straeter-Knowlen IM, Marks SL, Rishniw M, et al. Urethral pressure response to smooth and skeletal muscle relaxants in anesthetized, adult male cats with naturally acquired urethral obstruction. Am J Vet Res. 1995;56(7):919–923.
47. Sturgess CP, Hesford A, Owen H, et al. An investigation into the effects of storage on the diagnosis of crystalluria in cats. J Fel Med Surg. 2001;3(2):81–85.
48. Thumchai R, Lulich J, Osborne CA, et al. Epizootiologic evaluation of urolithiasis in cats: 3498 cases (1982–1992). J Am Vet Med Assoc. 1996;208:547–551.
49. Westropp JL, Buffington CA. Feline idiopathic cystitis: current understanding of pathophysiology and management. Vet Clin North Am Small Anim Pract. 2004;34:1043–1055.
50. Westropp JL, Kass PH, Buffington CA. Evaluation of stress in cats with idiopathic cystitis. Am J Vet Res. 2006;67(4):731–736.
51. Westropp JL, Kass PH, Buffington CA. In vivo evaluation of a2-adrenoceptors in cats with idiopathic cystitis. Am J Vet Res. 2007;68(2):203–207.
52. Wallius BM, Tidholm AE. Use of pentosan polysulphate in cats with idiopathic non-obstructive lower urinary tract disease: a double-blind randomised placebo-controlled trial. J Fel Med Surg. 2009;11:409–412.
53. Xu H, Laflamme DPL, Long GJ. Effects of dietary sodium chloride on health parameters in mature cats. J Fel Med Surg. 2009;11:435–441.