M. Lappin
The Kenneth W. Smith Professor in Small Animal Clinical Veterinary Medicine, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, CO, USA
Multiple vector-borne diseases can affect cats; those transmitted by ticks (multiple agents), fleas (multiple agents), mosquitoes (Dirofilaria immitis), and sandflies (Leishmania spp.) are among the most common. The Companion Animal Parasite Council (www.capcvet.org), European Scientific Counsel Companion Animal Parasites (www.esccap.org/guidelines/), and Companion Vector-Borne Diseases (www.cvbd.com) are excellent sources of information about vector-borne diseases in cats.
Most of the tick-borne diseases diagnosed in dogs have now been found in cats. Many of these tick-borne agents have been grown or amplified from blood or have induced serum antibodies in the serum of normal cats or those with clinical signs such as fever. In some countries, however, thorough evaluation of cats for tickborne disease agents has not been completed. In those situations, dog results can be used as evidence for the presence of individual agents in the region that could potentially infect cats. Results of studies from regional ticks can also be used as evidence for risk in cats.1-3
The purpose of this review is to provide an update on the diagnosis and management of feline tick-borne diseases of significance. Anaplasma phagocytophilum, Borrelia spp., Cytauxzoon spp., Ehrlichia spp., and Rickettsia spp. are discussed. It is less clear how important Hepatozoon spp. infections are in cats4 and how often Francisella tularensis infections are transmitted to cats by ticks and so they are not covered in depth.
Feline Granulocytotropic Anaplasmosis
Canine anaplasmosis has been recognized for many years. Cats have shown to be susceptible to A. phagocytophilum infection after experimental inoculation.5 The DNA of A. phagocytophilum has been amplified from blood of naturally exposed cats in multiple countries.6-11 The easiest way to remember the distribution of A. phagocytophilum infections in cats is to remember the range of Ixodes spp. or Lyme disease in people or dogs. In the United States, Ixodes scapularis transmits both A. phagocytophilum and B. burgdorferi, but some of the current evidence suggests that A. phagocytophilum is the more likely cause of the clinical and laboratory abnormities (Figure 1).
| Figure 1. Replete Ixodes scapularis adult ticks after a 7-day feeding period on an experimentally infested cat | 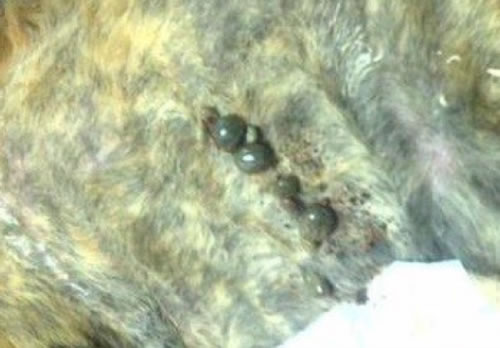
|
|
| |
While the pathogenesis of disease associated with A. phagocytophilum in cats is unknown, some cats experimentally inoculated with A. phagocytophilum developed anti-nuclear antibodies and increased interferon-gamma (IFN-γ) mRNA suggesting that an immune pathogenesis of disease may contribute to the clinical findings.12 Fever, anorexia, and lethargy are the most common clinical abnormalities in naturally infected cats.11 Whether this agent is associated with chronic recurrent fever in cats is unknown. In a recent experimental study, cats infected with A. phagocytophilum by exposure to wild-caught adult Ixodes scapularis from Rhode Island remained clinically normal over the 70-day study period despite being PCR positive for A. phagocytophilum DNA in blood for several weeks.5 In a larger unpublished study, we infested 10 cats with I. scapularis twice and induced A. phagocytophilum or Borrelia burgdorferi infection in all 10 cats.13 While repeated or new infections with both organisms occurred, all cats remained clinically normal. Both studies were performed using ticks from the same region, so it is possible a less pathogenic strain of the organism was present.14
Cats with fever in endemic areas can have blood smears examined cytologically, but morulae are not always detected in cats with clinical signs of anaplasmosis (Figure 2).
| Figure 2. Anaplasma phagocytophilum morulae (arrow) in the cytoplasm of a feline neutrophil noted after infestation of a cat with wild-caught Ixodes scapularis | 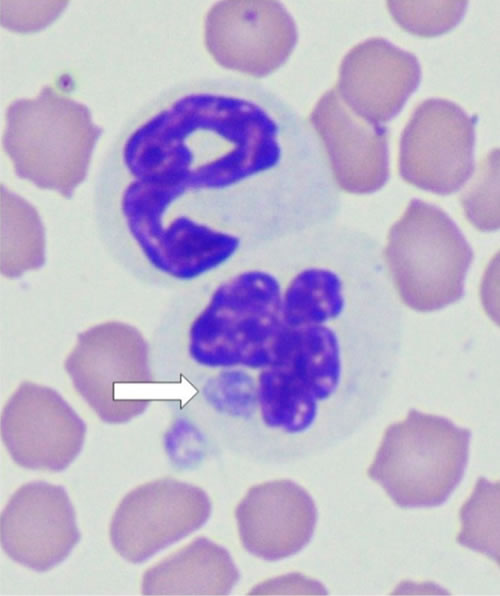
|
|
| |
Some commercial laboratories offer serologic testing or PCR assays to amplify A. phagocytophilum DNA from blood. In experimental infections, DNA is amplified from blood prior to seroconversion for most cats.5 Approximately 30% of cats with proven clinical infections induced by A. phagocytophilum are seronegative when first assessed serologically, but most of the proven cases evaluated to date have ultimately seroconverted. Therefore, cats with suspected anaplasmosis may need convalescent serum samples to prove infection. Alternatively, antibody testing could be combined with PCR testing of whole blood in acute cases. The SNAP 4DX Plus (IDEXX Laboratories) has been shown to be accurate for the detection of A. phagocytophilum antibodies in cats but is not labeled for this purpose.5 In addition, another peptide (P44-4) than the one used on the commercial assays detected antibodies even sooner.5
Several antibiotics have been administered to naturally infected cats, but most cats treated in the field become clinically normal within 24 to 48 hours after initiation of tetracycline or doxycycline administration and recurrence has not been reported in any cat to my knowledge.7,11 While clinically normal, the organism DNA can still be amplified from the blood of some cats, which suggests that treatment with tetracyclines for 21 to 30 days may be inadequate for eliminating the organism from the body. In one of our recent studies, the fact that an owner paid for a tick control product was not associated with decreased risk of having A. phagocytophilum antibodies in serum.15 These results suggest lack of compliance or lack of efficacy. As repeat new infections can occur, it is imperative to maintain tick control at all times, even in cats that have been previously infected.13
DNA homologous with A. platys has been amplified from the blood of cats in some countries with Rhipicephalus sanguineus.16,17 Further studies will be required to determine if disease associations exist with this agent in cats.
Feline Borreliosis
Borrelia burgdorferi is the cause of Lyme disease and is transmitted by Ixodes spp. Clinical illness in dogs and people is most common in the United States. While B. burgdorferi antibodies have been detected in the serum of cats for years, whether the agent induces illness in cats is still controversial.18-21
Recently, two groups have attempted to ascribe clinical illness to B. burgdorferi infection in cats.15,22 The cats that were positive for B. burgdorferi antibodies in Belgium, Sweden, and Germany had weakness, ataxia, and lameness as the most common clinical signs and doxycycline was apparently effective for treatment.22 The biggest limitation in that study was the failure to report results of assays for other feline disease agents that may be responsive to doxycycline, in particular A. phagocytophilum. The cats in Maine with suspected borreliosis were seropositive to B. burgdorferi C6 peptide but negative for A. phagocytophilum antibodies; had fever, weakness, lameness, lethargy, and inappetence as clinical signs; and had apparent responses to doxycycline.15 The biggest limitation in that study was the failure to perform A. phagocytophilum PCR or other diagnostic assays to evaluate for other feline disease agents that may be responsive to doxycycline. Recently, use of cefovecin was shown to be effective for the treatment of borreliosis in dogs.23 Whether this will prove to be true for cats remains to be determined. Currently there are no feline B. burgdorferi vaccines. In dogs, use of acaricides can block transmission of the agent and repeat infections can occur in cats.13,24 Thus, use of acaricides is imperative for the control of this agent.
Feline Cytauxzoonosis
Cats in the United States and Europe are infected by Cytauxzoon spp.4,25 Excellent review articles from European authors26 and American authors27 have recently been published. It is apparent that Cytauxzoon felis infections in the United States (transmitted by Amblyomma americanum) can be very pathogenic when compared with the Cytauxzoon spp. infections occurring in cats in other countries. This may represent different species in different countries;28 however, C. felis strain variations also play a role in whether clinical disease occurs within countries as well. For example, while fatal C. felis infections are common in some regions in the United States, cats that survive or have subclinical infections are also common.29,30 A recent study showed that C. felis could be transmitted between 36 and 48 hours of tick attachment and ingestion of A. americanum did not induce infections.31
In the United States, clinical infections are recognized most commonly in the spring, summer, and fall. Nonspecific complaints of lethargy and anorexia are reported frequently by owners. The infected cats have fever or hypothermia if presented in the final shock phase. Common physical examination findings that might lead to consideration of this agent as a differential diagnosis include pale mucous membranes, icterus, splenomegaly, and hepatomegaly. Discomfort and clinical evidence of central nervous system disease including seizures, tachypnea with or without respiratory distress, and sudden death on manipulation occur in some cats.
Piroplasms frequently can be seen on the erythrocytes but can be falsely negative in the acute stages of illness. The serious clinical signs of disease relate to the development of the schizonts in tissues. The syndrome can be diagnosed by cytologic demonstration of the piroplasms on erythrocytes; cytologic demonstration of schizonts in spleen, liver, or bone marrow samples; or by PCR of Cytauxzoon spp. DNA in blood or tissue aspirates.27
To date, clinically affected cats have the best response to the combination of azithromycin at 10 mg/kg, orally (PO), every 24 hours and atovaquone at 15 mg/kg, PO, every 8 hours32,33 with approximately 60% of treated cats responding. This combination is superior to diminazene or imidocarb protocols.32,34 Minimal restraint techniques should be used during administration of supportive care to lessen the likelihood of sudden death.
The poor overall treatment response in clinical cytauxzoonosis cases is a perfect example of why tick control can be so important. It is always better to prevent a vector-borne disease rather than attempt to treat it after illness has begun. Appropriate use of acaricides should lessen the risk of transmission of this agent.35
Feline Monocytotropic Ehrlichiosis
While canine ehrlichiosis is well characterized, less is known about the agents associated with disease in cats. It is likely that any country that has E. canis infections in dogs has E. canis infections in cats. Naturally exposed cats have been shown to have Ehrlichia-like bodies or morulae in peripheral lymphocytes or monocytes, have had DNA consistent with E. canis amplified from the blood or tissues, and have had antibodies that react to E. canis morulae or peptides in many countries.36-45 In two separate experimental studies, however, we have failed to amplify monocytotropic Ehrlichia spp. from blood or detect seroconversion in cats inoculated subcutaneously (SC) with different strains of cultured E. canis (Lappin and Breitschwerdt, unpublished observations, 2007; Lappin and Little, unpublished observations, 2010). These results indicate the E. canis-like DNA amplified from naturally infected cats may be from a different Ehrlichia spp. more infective to cats, not all E. canis strains will infect cats, not all cats are susceptible to infection by E. canis, or SC inoculation is not an effective method for infecting cats with E. canis. In addition, we have had field cases that have been positive for DNA identical to E. canis at two genes that never seroconverted.36 It is likely that cats at greater risk for Rhipicephalus sanguineous infestation are more likely to have higher prevalence rates for E. canis in countries like Brazil, where 9.4% of cats were PCR positive in one study.41 In Sicily, E. canis DNA was amplified from ticks collected from some cats.3
Fever, lethargy, and inappetence are commonly reported clinical abnormalities detected in cats with suspected ehrlichiosis and so testing may be indicated in these cats. Thrombocytopenia, anemia, and monocytosis appear to be the most common clinical laboratory findings in naturally infected cats. Almost every abnormality noted in dogs with clinical ehrlichiosis has been detected in cats including monoclonal gammopathy.42-46
A validated serologic assay is not currently available and some cats with E. canis-like DNA in blood were seronegative.36 Positive serologic test results occur in both healthy and clinically ill cats, and so a diagnosis of clinical ehrlichiosis should not be based on serologic test results alone. Ehrlichia spp. PCR and gene sequencing can be used to confirm infection and should be considered the tests of choice at this time.
Clinical improvement after therapy with tetracycline, doxycycline, or imidocarb dipropionate was reported for most cats with suspected monocytotropic ehrlichiosis. For some cats, however, a positive response to therapy was a criterion for the diagnosis of ehrlichiosis. The current recommendation of the ACVIM Infectious Disease Study Group (www.acvim.org) is to administer doxycycline (10 mg/kg PO q24h or 5 mg/kg PO q12h for 28 days). For cats with treatment failure or those intolerant of doxycycline, imidocarb dipropionate can be administered [5 mg/kg intramuscularly (IM) or SQ twice, 14 days apart]. Salivation and pain at the injection site are the common adverse effects and imidocarb efficacy is in question for the treatment of canine monocytotropic ehrlichiosis. Pancytopenia occurs in cats with ehrlichiosis and when it occurs in dogs, it may not respond to treatment;36 this is another example of why acaricides should be used to attempt to avoid infection with vectorborne disease agents.
Feline Tick-Borne Rickettsiosis
Rickettsia spp. are obligate intracellular gram-negative bacteria that are divided into the spotted fever group and the typhus group. In the United States, cats can be infected by Rickettsia felis and have been shown to have antibodies against R. rickettsii, which is tick-borne.47 In Spain, R. conorii and R. massiliae antibodies were found in cat serum and DNA amplified from cat blood, suggesting that cats could play a role in the life cycles of these agents or be clinically affected.48 In one study of cats with fever, we showed R. felis and R. rickettsii antibody prevalence rates in cats in the United States to be 5.6% and 6.6%, respectively, but DNA of neither organism was amplified from blood.47 These results prove that cats are sometimes exposed to spotted fever group organisms, but further data are needed to determine significance of diseases associations. Because clinical illness in cats from spotted fever organisms has not been documented, optimal treatment is unknown. Base d on results in dogs with R. rickettsii infection however, doxycycline or a fluoroquinolone would be logical choices. The evidence for spotted fever agents in cats in the United States and Europe provides further evidence that acaricides should be used in cats as these agents can cause zoonotic infection in humans.
Conclusion
Tick control is warranted for cats as well as dogs. Products with efficacy against fleas should also be used because fleas can be vectors for several Bartonella spp., potentially the hemoplasmas, potentially Coxiella burnetii, (Cypress), R. felis, and Yersinia pestis.
References
1. Skarphédinsson S, Lyholm BF, Ljungberg M, et al. Detection and identification of Anaplasma phagocytophilum, Borrelia burgdorferi, and Rickettsia helvetica in Danish Ixodes ricinus ticks. APMIS. 2007;115:225–230.
2. Smith FD, Wall LE. Prevalence of Babesia and Anaplasma in ticks infesting dogs in Great Britain. Vet Parasitol. 2013;198:18–23.
3. Pennisi MG, Persichetti MF, Serrano L, et al. Ticks and associated pathogens collected from cats in Sicily and Calabria (Italy). Parasit Vectors. 2015;8:512.
4. Díaz-Regañón D, Villaescusa A, Ayllón T, et al. Molecular detection of Hepatozoon spp. and Cytauxzoon sp. in domestic and stray cats from Madrid, Spain. Parasit Vectors. 2017;10:112.
5. Lappin MR, Chandrashekar R, Stillman B, et al. Evidence of Anaplasma phagocytophilum and Borrelia burgdorferi infection in cats alter exposure to wildcaught adult Ixodes scapularis ticks. J Vet Diagn Invest. 2015;27:522–525.
6. Bjöersdorff A, Svendenius L, Owens JH, Massung RF. Feline granulocytic ehrlichiosis - a report of a new clinical entity and characterisation of the infectious agent. J Small Anim Pract. 1999;40:20–4.
7. Lappin MR, Breitschwerdt EB, Jensen WA, et al. Molecular and serologic evidence of Anaplasma phagocytophilum infection in cats in North America. J Am Vet Med Assoc. 2004;225:893–896.
8. Adaszek Ł, Góma M, Skrzypczak M, et al. Three clinical cases of Anaplasma phagocytophilum infection in cats in Poland. J Feline Med Surg. 2013;15:333–337.
9. Bergmann M, Englert T, Stuetzer B, et al. Prevalence of selected rickettsial infections in cats in Southern Germany. Comp Immunol Microbiol Infect Dis. 2015;42:33–36.
10. Lee SH, VanBik D, Kim NH, et al. First molecular detection and genetic analysis of Anaplasma phagocytophilum in shelter cats in Seoul, Korea. Infect Genet Evol. 2016 Oct 29. pii: S1567-1348(16)30434-8.
11. Savidge C, Ewing P, Andrews J, et al. Anaplasma phagocytophilum infection of domestic cats: 16 cases from the northeastern USA. J Feline Med Surg. 2016;18:85–91.
12. Foley JE, Leutenegger CM, Dumler JS, Pedersen NC, Madigan JE. Evidence for modulated immune response to Anaplasma phagocytophila sensu lato in cats with FIV-induced immunosuppression. Comp Immunol Microbiol Infect Dis. 2003;26:103–113.
13. Lappin MR, Huesken R, Stanneck D. Anaplasma phagocytophilum and Borrelia burgdorferi infections in cats exposed twice to Ixodes scapularis. Parasit Vectors. 2017, in review:
14. Rejmanek D, Freycon P, Bradburd G, et al. Unique strains of Anaplasma phagocytophilum segregate among diverse questing and non-questing Ixodes tick species in the western United States. Ticks Tick Borne Dis. 2013;4:482–487.
15. Hoyt K, Chandrashekar R, Beall M, et al. Evidence for clinical borreliosis and anaplasmosis in cats in Maine. J Feline Med Surg. 2017, in review.
16. Lima MLF, Soares PT, Ramos CAN, et al. Molecular detection of Anaplasma platys in a naturally-infected cat in Brazil. Braz J Microbiol. 2010;41:381–385.
17. Qurollo BA, Balakrishnan N, Cannon CZ, et al. Co-infection with Anaplasma platys, Bartonella henselae, Bartonella koehlerae and 'Candidatus Mycoplasma haemominutum' in a cat diagnosed with splenic plasmacytosis and multiple myeloma. J Feline Med Surg. 2014;16:713–20.
18. Burgess EC, et al. Experimentally induced infection of cats with Borrelia burgdorferi. Am J Vet Res. 1992;553:1507–1511.
19. Levy SA, O'Connor TP, Hanscom JL, et al. Evaluation of a canine C6 ELISA Lyme disease test for the determination of the infection status of cats naturally exposed to Borrelia burgdorferi. Vet Ther. 2003;4:172–177
20. Magnarelli LA, Bushmich SL, IJdo JW, et al. Seroprevalence or antibodies against Borrelia burgdorferi and Anaplasma phagocytophilum in cats. Am J Vet Res. 2005;66:1895–1899.
21. Krupka I, Straubinger RK. Lyme borreliosis in dogs and cats: background, diagnosis, treatment and prevention of infections with Borrelia burgdorferi sensu stricto. Vet Clin North Am Small Anim Pract. 2010;40:1103–1119.
22. Pantchev N, Vrhovec MG, Pluta S, Straubinger RK. Seropositivity of Borrelia burgdorferi in a cohort of symptomatic cats from Europe based on a C6-peptide assay with discussion of implications in disease aetiology. Berl Munch Tierarztl Wochenschr. 2016;129:333–339.
23. Wagner B, Johnson J, Garcia-Tapia D, et al. Comparison of effectiveness of cefovecin, doxycycline, and amoxicillin for the treatment of experimentally induced early Lyme borreliosis in dogs. BMC Vet Res. 2015;11:163.
24. Honsberger NA, Six RH, Heinz TJ, et al. Efficacy of sarolaner in the prevention of Borrelia burgdorferi and Anaplasma phagocytophilum transmission from infected Ixodes scapularis to dogs. Vet Parasitol. 2016;222:67–72.
25. Carli E, Trotta M, Chinelli R, et al. Cytauxzoon sp. infection in the first endemic focus described in domestic cats in Europe. Vet Parasitol. 2012;183:343–352.
26. Lloret A, Addie DD, Boucraut-Baralon C, et al. European Advisory Board on Cat Diseases. Cytauxzoonosis in cats: ABCD guidelines on prevention and management. J Feline Med Surg. 2015;17:637–641.
27. Sherrill MK, Cohn LA. Cytauxzoonosis: diagnosis and treatment of an emerging disease. J Feline Med Surg. 2015;17:940–948.
28. Gallusová M, Jirsová D, Mihalca AD, et al. Cytauxzoon infections in wild felids from Carpathian-Danubian-Pontic Space: further evidence for a different Cytauxzoon species in European felids. J Parasitol. 2016;102;377–380.
29. Meinkoth J, Kocan AA, Whitworth L, et al. Cats surviving natural infection with Cytauxzoon felis: 18 cases (1997–1998). J Vet Intern Med. 2000;14:521–525.
30. Rizzi TE, Reichard MV. Cohn LA, et al. Prevalence of Cytauxzoon felis infection in healthy cats from enzootic areas in Arkansas, Missouri, and Oklahoma. Parasit Vectors. 2015;8:13.
31. Thomas JE, Ohmes CM, Payton ME, et al. Minimum transmission time or Cytauxzoon felis by Amblyomma americanum to domestic cats in relation to duration of infestation, and investigation of ingestion of infected ticks as a potential route of transmission. J Feline Med Surg. 2017 Feb 1:1098612X17691172. doi: 10.1177/1098612X17691172. [Epub ahead of print]
32. Cohn LA, Birkenheuer AJ, Brunker JD, et al. Efficacy of atovaquone and azithromycin or imidocarb dipropionate in cats with acute cytauxzoonosis. J Vet Intern Med. 2011;25:55–60.
33. Schreeg ME, Marr HS, Tarigo J, et al. Pharmacogenomics of Cytauxzoon felis cytochrome b: implications for atovaquone and azithromycin therapy in domestic cats with cytauxzoonosis. J Clin Microbiol. 2013;51:3066–3069.
34. Lewis KM, Cohn LA, Marr HS, Birkenheuer AJ. Failure of efficacy and adverse events associated with dose-intense diminazene diaceturate treatment of chronic Cytauxzoon felis infection in five cats. J Feline Med Surg. 2014;16:157–163.
35. Reichard MV, Thomas JE, Arther RG, et al. Efficacy of an imidacloprid 10%/flumethrin 4.5% collar (Seresto®, Bayer) for preventing the transmission of Cytauxzoon felis to domestic cats by Amblyomma americanum. Parasitol Res. 2013;112(Suppl 1):11–20.
36. Breitschwerdt EB, Abrams-Ogg AC, Lappin MR, et al. Molecular evidence supporting Ehrlichia canis-like infection in cats. J Vet Intern Med. 2002;16:642–649.
37. Aguirre E, Tesouro MA, Amusategui I, et al. Assessment of feline ehrlichiosis in central Spain using serology and a polymerase chain reaction technique. Ann N Y Acad Sci. 2004;1026:103–135.
38. Ayllón T, Diniz PP, Breitschwerdt EB, et al. Vector-borne diseases in client-owned and stray cats from Madrid, Spain. Vector Borne Zoonotic Dis. 2012;12:143–150.
39. Solano-Gallego L, Hegarty B, Espada Y, Llull J, Breitschwerdt E. Serological and molecular evidence of exposure to arthropod-borne organisms in cats from northeastern Spain. Vet Microbiol. 2006;118:274–277.
40. Tabar MD, Altet L, Francino O, et al. Vector-borne infections in cats: molecular study in Barcelona area (Spain). Vet Parasitol. 2008;151:332–336.
41. Braga ÍA, dos Santos LG, de Souza Ramos DG, et al. Detection of Ehrlichia canis in domestic cats in the central-western region of Brazil. Braz J Microbiol. 2014;45:641–645.
42. Bouloy RP, Lappin MR, Holland CH, et al. Feline ehrlichiosis: clinical case and serologic survey. J Am Vet Med Assoc. 1994;204:14 75–1478.
43. Peavy GM, Holland CJ, Dutta SK, et al. Suspected Ehrlichia infection in a household of cats. J Am Vet Med Assoc. 1997;210:231–234.
44. Beaufils JP, Martin-Granel J, Jumelle P, et al. Ehrlichiosis in cats. A retrospective study of 21 cases. Pratique Médicale Chirurgicale de l'Animal de Compagnie. 1999;34:587–596.
45. Braga IA, dos Santos LG, Melo AL, et al. Hematological values associated to the serological and molecular diagnostic in cats suspected of Ehrlichia canis infection. Rev Bras Parasitol Vet. 2013;22:470–474.
46. Neer TM, Breitschwerdt EB, Greene RT, Lappin MR. Consensus statement on ehrlichial disease of small animals from the Infectious Disease Study Group of the ACVIM. J Vet Intern Med. 2002;16:309–315.
47. Bayliss DB, Morris AK, Horta MC, et al. Prevalence of Rickettsia species antibodies and Rickettsia species DNA in the blood of cats with and without fever. J Feline Med Surg. 2009;11:266–270.
48. Segura F, Pons I, Miret J, et al. The role of cats in the eco-epidemiology of spotted fever group diseases. Parasit Vectors. 2014;7:353.