One aspect of finding the right therapeutic for any condition is choosing the most appropriate technology platform. Molecules used as active substances can be divided into two classes: small and large molecules. They differ not only in terms of size, but also in how they are made, how they behave, their mode of action in the body, and their suitability for certain drug forms.
Small, chemically manufactured molecules make up over 90% of the drugs on the market today and, for the most part, act intracellularly. By contrast, large molecules, also known as biologics, are therapeutic proteins that have extracellular targets. Biologic or biological therapy uses portions of the body’s natural immune system to treat a disease. Therapeutic biologics are proteins derived from biological sources, unlike chemically synthesized “small molecule” pharmaceuticals. Over time veterinary medicine will see development of new therapeutics that are highly targeted, safe, and have minimal or no side effects.
Key Facts
- Some of the oldest forms of biologics are extracted from the bodies of animals, including humans. These include:
- Whole blood and other blood components
- Organs and tissue transplants
- Stem cell therapy
- Antibodies for passive immunization (e.g., to treat a virus infection)
- Colostrum
- Fecal microbiota
- Reproductive cells
- Some biologics that were previously extracted from animals, such as insulin, are now more commonly produced by recombinant DNA.
- Monoclonal antibodies (mAbs) are laboratoryproduced antibodies used to treat many different human inflammatory conditions and cancers, similar to the antibodies that the natural immune system uses to fight off bacteria and viruses.
- Because monoclonal antibodies are “custom designed,” they can be made to target specific proteins, such as cellular receptors or soluble molecules involved in disease pathogenesis.
How Does Biologic Therapy Compare with Conventional Drug Therapy?
Biologic therapy works by targeting proteins that are a part of the pathophysiology of an inflammatory disease, aid in the growth of cancer cells, or are specific molecules on cancer cells that can destroy the cells. Therapeutic monoclonal antibodies, interferon, interleukin-2 (IL-2), and several types of colony stimulating factors (CSF, GM-CSF, G-CSF) are forms of biological therapy.
Monoclonal Antibodies
Monoclonal antibodies, or mAbs, are laboratory produced antibodies. They are a common type of biologic therapy used to treat many different inflammatory conditions and cancers in humans. These are similar to the antibodies that the immune system uses to fight off bacteria and viruses, but they are “custom designed” and can therefore be made specifically to selectively target proteins, such as cellular receptors or soluble molecules involved in disease pathogenesis.
Modes of biologic therapy that involve blocking the action of specific proteins of inflammation (e.g., tumor necrosis factor [TNF]) are being used for the treatment of a number of diseases in humans, including rheumatoid arthritis and Crohn’s disease. Adalimumab (Humira®) and infliximab (Remicade®) are examples of commercially available injectable TNF-blocking treatments for patients with severe rheumatoid arthritis (Figure 1A). Other mAbs, such as dupilumab, target the receptors for cytokines, which modulate the signaling for IL-4 and IL-13 and will be used to treat allergic asthma and atopic dermatitis in humans (Figure 1B).
| Figure 1 | 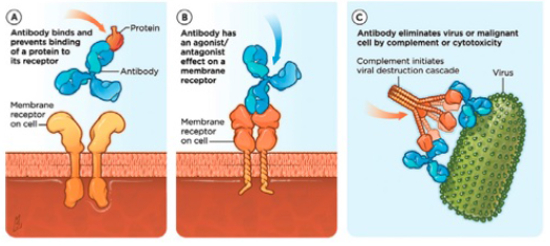
(A) One mode of monoclonal antibody therapy blocks the proteins that cause inflammation by injection of tumor necrosis factor (TNF), thereby preventing a protein from binding to its receptor. (B) Another mode targets the cytokine receptors to inhibit the signaling g of IL-4 and IL-13. |
|
| |
Safety of Monoclonal Antibody Therapy
The side effects of biological therapy depend on the type of treatment and the target of the therapy. In people, side effects can include flu-like symptoms, such as chills, fever, muscle aches, weakness, loss of appetite, nausea, vomiting, and diarrhea. Some patients develop a rash, and some bleed or bruise easily. All of these depend on the target of the mAb. The long-term side effects of the various currently available biological therapies will be better defined with future research from which will also surely emerge new and valuable forms of these treatments. This compares with traditional pharmaceuticals, which have a real risk for safety due to overdose; concomitant drug interactions; underlying conditions; or contraindications, such as species or age.
An additional difference between traditional small molecule pharmaceuticals and mAbs is the way that they are metabolized and eliminated from the body. Small molecules are metabolized by the liver or intestines and eliminated by the kidneys or gastrointestinal tract. In contrast, mAbs are eventually eliminated via intracellular catabolism in the lysosome, where they are broken down into peptides or amino acids that can be either reused for synthesis of new proteins or renally excreted. Those mAbs in circulation that bind to the neonatal receptor on endothelial cells are protected from degradation such that they are recycled back into the plasma. This is part of the reason for their long half-life.
For all of these reasons, mAbs are relatively safe to use in treating people and animals with co-morbidities, such as liver disease; concurrently with many other pharmaceuticals; and in animals of any age.
How Are Monoclonal Antibodies (mAbs) Made?
To create mAbs, researchers inject mice with an antigen from a human or animal. They then harvest the antibodyproducing cells from the mice and individually fuse them with a myeloma cell (cancerous B cell) to produce a fusion cell known as a hybridoma. Each hybridoma then divides to produce identical daughter cells or clones hence the term “monoclonal” - and antibodies secreted by different clones are tested to identify the antibodies that bind most strongly to the antigen, have the longest half-life, etc. Large quantities of antibodies can be produced by these immortal hybridoma cells.
Because murine antibodies can themselves elicit an immune response in humans or other animals, which would reduce their effectiveness, the murine antibodies are often speciated (humanized, caninized, etc.) by replacing as much of the mouse portion of the antibody as possible with portions from the target species. This is done through genetic engineering (Figure 2).
| Figure 2 | 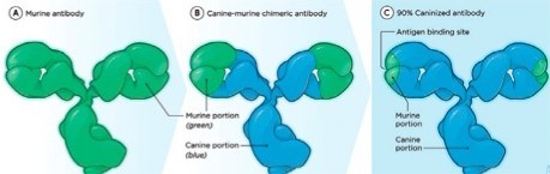
Through genetic engineering, the murine antibodies that can actually elicit an immune response and reduce mAb effectiveness are speciated by replacing as much as possible with a portion from the target species (e.g., canine) |
|
| |
Some mAbs stimulate an immune response that destroys cancer cells. Similar to the antibodies produced naturally by B cells, these mAbs “coat” the cancer cell surface, triggering its destruction by the immune system. U.S. FDA-approved mAbs of this type include rituximab, which targets the CD20 antigen found on non-Hodgkin lymphoma cells, and alemtuzumab, which targets the CD52 antigen found on B-cell chronic lymphocytic leukemia (CLL) cells. Rituximab may also trigger cell death (apoptosis) directly.
Monoclonal Antibody Therapy in Veterinary Medicine
There are currently (as of November 2016) three monoclonal antibodies approved for use in veterinary medicine in the United States, and they are all licensed by the U.S. Department of Agriculture (USDA) for use in dogs. Blontress® targets CD20 on lymphocytes and aids in the treatment of dogs with B-cell lymphoma. Tactress® targets CD52 on T lymphocytes and is indicated as an aid in the treatment of dogs with T-cell lymphoma. Both of these products are manufactured by Aratana Therapeutics.
Cytopoint™ is a monoclonal antibody that reduces clinical signs of atopic dermatitis (AD) in dogs. It targets the circulating cytokine canine interleukin-31 (IL-31), which has been shown to be elevated in dogs with AD. IL-31 has been demonstrated to cause pruritus in dogs; and inhibition, both in laboratory dogs and client-owned dogs with atopic dermatitis, controls both pruritus and inflammation. Cytopoint is manufactured by Zoetis.
Nexvet Biopharma has completed and published information on two different monoclonal antibodies targeted at the inhibition of nerve growth factor (NGF). These work by inhibiting NGF, which acts on pain-sensing nerve fibers to increase their excitability and increase the sprouting of new nerve fibers into inflamed tissues. NGF has been found to be elevated in the joints of dogs with osteoarthritis. Ranevetmab will be used to control pain associated with canine osteoarthritis. Frunevetmab is being developed as a monthly subcutaneous injectable for the control of pain associated with osteoarthritis in cats.
In the future, veterinary medicine will see the development of additional therapeutic mAbs for companion animals and other species. These highly targeted and safe therapeutics are likely to prove beneficial to treat diseases uniquely without the side effects associated with traditional broad-spectrum pharmacotherapy.
References
1. Bammert G, Dunkle B, Fici G, et al. Identification and characterization of anticanine interteukin-31 neutralizing monoclonal antibodies (abstract). Vet Dermatol. 2014;25:404.
2. Dunham S, Teel J, Bammert G, et al. Evaluation of anti-lL-31 monoclonal antibodies in a model of IL-31-inducedpruritus in Beagle dogs (abstract). Vet Dermatol. 2014;25:403.
3. Gearing DP, Huebner M, Virtue ER, et al. In vitro and in vivo characterization of a fully felinized therapeutic anti-nerve growth factor monoclonal antibody for the treatment of pain in cats. J Vet Intern Med. 2016;30(4):1129–1137.
4. Gearing DP, Virtue ER, Geanng RP, et al. A fully caninised anti-NGF monoclonal antibody for pain relief in dogs. BMC Vet Res. 2013;9:226.
5. Gonzales AJ, Humphrey WR, Messamore JE. lnterfeukin-31: its role in canine pruritus and naturally occurring canine atopic dermatitis. Vet Dermatol. 2013;24(1):48–e12.
6. Gruen ME, Thomson AE, Griffith EH, et al. A feline-specific anti-nerve growth factor antibody improves mobility in cats with degenerative joint disease-associated pain: a pilot proof of concept study. J Vet Int Med. 2016;30(4):1138–1148.
7. Michels GM, Ramsey DS, Watsh KF, et al. (2016) A blinded, randomized, placebo-controlled dose determination trial of lokivetmab (ZTS-00103289), a caninized, anti-canine IL-31 monoclonal antibody in client owned dogs with atopic dermatitis. Vet Dermatol. 2016;27:478–e129.
8. Olivry T, Bainbridge G. Advances in veterinary medicine: therapeutic monoclonal antibodies for companion animals. Clinician’s Brief 10 Mar 2015. www.itchcycle.com/antibodytherapy.