D. Hutchinson, DVM, DACVN
In 2010 eight percent of the world's human population were 65 years of age or older. By 2050 this number is expected to triple to 16% of the world's population.1 With the growing human geriatric population has come increasing interest in diseases commonly afflicting geriatrics as well as unavoidable age-related syndromes impacting quality of life and ability to live independently. Like humans, pets are also aging and there is increased interest in how to improve their quality and quantity of life. One syndrome speculated to affect all aging individuals (human, canine and otherwise) is sarcopenia, the age-related loss of lean body mass (LBM) in the absence of disease. Sarcopenia encompasses both a loss of muscle mass and function. Furthermore, many geriatrics also suffer from chronic diseases such as chronic kidney disease (CKD) which occurs in 32% of cats 15 years of age or older.2 Such diseases also impact LBM through mechanisms involved in cachexia, which is muscle loss due to disease. Together, sarcopenia and cachexia represent important syndromes impacting quality and quantity of life in much of our geriatric population.
Importance
Sarcopenia occurs in all aging individuals. In humans, there is loss of 5% of muscle mass per decade of life from the fourth decade onwards, potentially increasing after the age of 65 years.3 The rate of muscle loss in the aging pet population has yet to be investigated, but is likely much higher due to their relatively shorter lifespan. Sarcopenia is a newly emerging area of interest in veterinary medicine; however, it is a topic of extensive research within the human medical field due to impact on quality of life, as well as its direct association with morbidity and ability to live independently. Similarly, the veterinary profession has begun to consider the effect of age-related muscle loss on the quality of life in our patients. This is particularly of interest in pets already suffering from muscle loss due to chronic disease such as CKD or congestive heart failure (CHF). Many of these pets have lost significant LBM, impacting their mobility, quality of life, and likely mortality - in humans suffering from disease it has been demonstrated that cachexia is an independent predictor of mortality.4 While yet to be investigated, it is likely that cachexia indirectly impacts survival time in pets. Cachexia results in several negative outcomes associated with quality of life, which plays a pivotal role in clients' choice of euthanasia. Addressing causes of muscle loss in aging, diseased patients is therefore of great interest. This has led to the creation of muscle condition scoring (MCS) systems for dogs and cats designed for use in medical records. In order to understand how to manage these syndromes, it is important to first grasp the pathophysiologic mechanisms involved.
Mechanisms
Sarcopenia and cachexia are both multifactorial syndromes and the underlying mechanisms involved in each overlap considerably (Figure 1). Muscle mass results from the balance between anabolic and catabolic pathways involved in protein synthesis or breakdown, respectively. Both pathways are impacted negatively in cachectic and sarcopenic individuals. Aging, as well as chronic disease, may result in decreased concentrations, and/or signaling of hormones critical to protein synthesis including insulin-like growth factor-1 (IGF-1) and growth hormone (GH) as well as insulin resistance. Cachectic individuals also have increased concentrations of cortisol and adrenergic hormones resulting in increased fat oxidation, insulin resistance and hypermetabolism.5 Inflammatory mediators [e.g., interleukin (IL)-1β, 6, and tumor necrosis factor alpha (TNF-α) through activation of the ubiquitin-proteasome pathway via NF-κB-dependent and independent mechanisms) are increased in cachectic individuals and contribute to decreased food intake and protein catabolism. These cytokines also contribute to insulin resistance as well as other mechanisms that impact protein synthesis such as inhibition of GH and IGF-1. An observational study in more than 2,000 men and women showed an association between TNF-α and decline in muscle mass and strength.6
| Figure 1. Overlapping mechanisms of cachexia and sarcopenia | 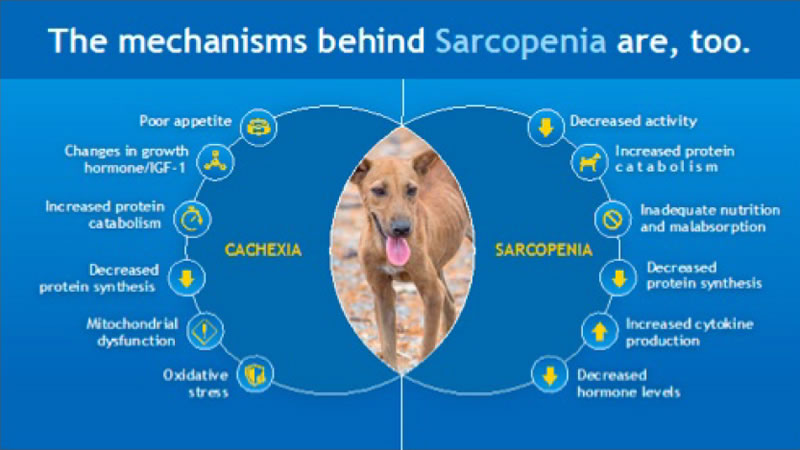
|
|
| |
Mitochondrial efficiency is also compromised with age and disease and is thought to contribute to decreased muscle mass through a number of mechanisms. Oxidative stress has been well documented in humans suffering from chronic disease and is believed to play an important role in protein catabolism. In part this may result from stimulation of the ubiquitin-proteasome system and lower activity of antioxidant enzymes including superoxide dismutase and glutathione peroxidase as well as inhibition of protein synthesis.7 Similar mechanisms related to oxidative stress are likely involved in degenerative age-related conditions including sarcopenia as expressed in the Free Radical Theory on Aging (also known as the Oxidative Stress Theory of Aging). As outlined above, many factors involved in cachexia and sarcopenia have been identified in humans and further research is needed to understand any differences that may exist in mechanisms involved in muscle loss in dogs and cats.
Management
While there is no single therapy available that will halt cachexia or sarcopenia, a multimodal approach, including pharmacologic and nutritional intervention in addition to exercise programs, may minimize muscle loss due to these syndromes. And in the case of cachexia, addressing the underlying disease is imperative for minimizing loss of LBM.
Several novel pharmacologic therapies are currently undergoing trials for use in both cachectic and sarcopenic humans and may serve as a starting point for therapeutic opportunities that may benefit veterinary patients in the future but are beyond the scope of this review.
In humans, both resistance and aerobic exercise have been shown to be effective in minimizing muscle loss and improving muscle function, as well as attenuating inflammatory markers, reducing fatigue, and improving quality of life in geriatric and cachectic patients suffering from loss of LBM.8 These actions are thought to be due to anabolic effects of exercise including stimulation of IGF-1, inhibition of inflammatory mediators and myostatin, as well as increased antioxidant capacity through enhancement of free radical scavenging enzymes and other mechanisms. While instituting such programs poses unique challenges in veterinary patients, it is possible and likely would be enjoyed by the majority of canine patients. Simple plans could include increasing normal daily activities of a geriatric canine patient (short walks, hikes, and playing catch) and may improve their agility and attenuate muscle loss. Cats can often be encouraged to exercise through play activities such as replicating the hunt experience, utilizing stairs and laser pointers. Many geriatric dogs and cats suffer from degenerative joint disease (DJD), which may impact their level of activity; this can be effectively managed through nutritional interventions (therapeutic joint diets, omega-3 (n-3) fatty acid supplementation) as well as judicious pharmacologic pain management. Addressing DJD often greatly enhances self-initiated exercise in geriatric dogs and cats and may assist in improving quality of life, mobility, and muscle mass/function with little cost or risk to the family. It is critical that the veterinary team address such conditions when instituting plans to increase a geriatric patient's activity.
Nutrition holds great potential for combating many of the mechanisms involved in both cachexia and sarcopenia. Inflammation is believed to play a fundamental role in development of age- and disease-related muscle loss, as outlined above. Long-chain n-3 fatty acids have demonstrated anti-inflammatory effects in both humans and dogs and cats and therefore may assist in attenuating the inflammatory component that is so fundamental in both of these syndromes. On the veterinary side, n-3 fatty acids have also been shown to improve appetite and reduce muscle loss in dogs with heart failure.9
Antioxidants and mitochondrial cofactors may also be beneficial as part of multimodal therapy for muscle wasting syndromes, due to the pivotal role that oxidative stress plays in their pathogenesis. While efficacy of antioxidants appears promising for patients with cachexia, optimal dosage, route of administration or the most effective combinations have not been established, and studies in veterinary patients at this point in time are lacking.
In veterinary patients suffering from muscle loss, it is also critical that caloric and protein requirements are met daily. This is an issue plaguing CKD patients. While renal therapeutic foods have historically been characterized as unpalatable, recent studies found that 94% of cats and 97% of dogs with CKD successfully transitioned to Prescription Diet k/d.10,11 Nonetheless, hyporexia (decreased food intake) is quite common in the CKD patient. In the case of insufficient caloric intake, muscle will ultimately be catabolized to provide a necessary source of energy. Patients eating insufficient calories are often also not meeting their protein requirements, further perpetuating muscle loss. While studies focused on supplementing protein or specific amino acids in humans have shown mixed benefits, it is generally agreed that meeting the patient's basic need for nitrogen and the essential amino acids is imperative. Since insufficient caloric and protein intake further exacerbates muscle wasting in cachectic pets, it must be prevented when possible through meticulous use of antiemetics, appetite stimulants, proper feeding orders, and when necessary and appropriate, enteral nutritional interventions such as esophagostomy tube placement. A thorough nutritional assessment as part of every patient visit greatly reduces the risk of malnutrition in both healthy and diseased senior pets.
Table 1. Non-pharmacologic multimodal management of cachexia and sarcopenia
|
Minimize muscle loss
|
Meet nutrient needs
|
Maximize muscle synthesis
|
|
• Maximize treatment of underlying conditions
• Omega-3 fatty acids from fish oil
• Targeted levels of L-carnitine
|
• Ensure caloric needs are met daily
• Meet essential amino acid needs
• Consider anti-emetics, appetite stimulants
• Consider feeding tubes in consistently anorexic patients
|
• Maximize quality of life to keep pets moving
• Targeted levels of L-carnitine
|
While there is no single therapy shown to mitigate muscle loss associated with aging and disease, a multimodal approach including nutritional and exercise therapies can provide meaningful benefits that improve quantity and quality of life in our aging, diseased patients. The human medical community has made great strides towards better understanding and slowing these clinically important syndromes. As a profession, veterinarians are charged with following this exploration into these critical, devastating syndromes and have taken the first steps through creation of muscle condition scoring systems and pilot studies documenting that such syndromes indeed exist in our patient population.
References
1. National Institute on Aging. Global Health and Aging. Available at: www.who.int/ageing/publications/global_health.pdf. Accessed Feb 28, 2017.
2. IDEXX Laboratories, Data on File 2017.
3. Forbes GB, Reina JC. Adult lean body mass declines with age: some longitudinal observations. Metabolism. 1970;19:653–663.
4. Tan BH, Fearon KC. Cachexia: prevalence and impact in medicine. Curr Opin Clin Nutr Metab Care. 2008;11:400–407.
5. Ali S, Garcia JM. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options - a min-review. Gerontology. 2014;60(4):294–305.
6. Schaap LA, Pluijm SM, Deeg DJ, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64(11):1183–1189.
7. Mantovani G, Maccio A, Madeddu C, et al. Antioxidant agents are effective in inducing lymphocyte progression through cell cycle in advanced cancer patients: assessment of the most important laboratory indexes of cachexia and oxidative stress. J Mol Med. 2003;81:664–673.
8. Little JP, Phillips SM. Resistance exercise and nutrition to counteract muscle wasting. Appl Physiol Nutr Metab. 2009;34:817–828.
9. Freeman LM, Rush JE, Kehayias JJ, et al. Nutritional alterations and the effect of fish oil supplementation on dogs with heart failure. J Vet Intern Med. 1998;12(6):440–448.
10. Fritsch DA, Jewell DE. Acceptance and effects of a therapeutic renal food in pet cats with chronic kidney disease. Vet Rec Open. 2015;2:e000128.
11. Hall JA, Fritsch DA, Yerramilli M, et al. A longitudinal study on the acceptance and effects of a therapeutic renal food in pet dogs with IRIS-Stage 1 chronic kidney disease. J Anim Physiol Anim Nutr. 2017; In Press.