R. Loh
Introduction
Fish rely on the qualities of water for many biological processes, and their health depends on several key parameters that include water temperature, ammonia (NH3), nitrite (NO2-), nitrate (NO3-), pH, carbonate hardness (KH, also known as alkalinity), general hardness (GH, also known as permanent hardness), dissolved oxygen (DO), and salinity. This part of the workshop gives an overview of each water parameter as it applies to fish health, and practical ways to correct water-quality problems.
There are more than 40 thousand species of fish, and each fish species has specific environmental requirements to live (e.g., temperature, salinity, pH, etc.). Understanding these requirements is necessary to provide good diagnosis and management recommendations.
Nitrogen Cycle and Biofilters
Fish continually produce ammonia as their waste product, and at high levels it can be toxic to fish. The biofilter provides a physical substrate for beneficial bacteria to colonise so they can detoxify fish wastes. The conversion of ammonia to nitrite (predominantly by Nitrosomonas, Nitrospira, and Nitrosococcus) and from nitrite to nitrate (predominantly by Nitrobacter, Nitrococcus, and Nitrospina) is termed the "nitrogen cycle." In the natural environment, the end product (nitrate) will be incorporated into plants/algae. However, in an aquarium without plants, nitrate will accumulate unless it is removed by partial water changes or by using a denitrification unit.
Several factors affect the performance of the biofilter, and they include:
 Temperature (works between 12–58°C; most efficient between 28–36°C)
Temperature (works between 12–58°C; most efficient between 28–36°C)
 Dissolved oxygen (DO below 80% saturation will decrease biofilter efficiency and processes will cease if DO levels fall below 2 mg/L)
Dissolved oxygen (DO below 80% saturation will decrease biofilter efficiency and processes will cease if DO levels fall below 2 mg/L)
 The pH of the water (reactions occurring faster between a pH of 8–9, but is impeded at pH <5)
The pH of the water (reactions occurring faster between a pH of 8–9, but is impeded at pH <5)
 Salinity (reactions occur more slowly at higher salinities, hence the reason it takes 8 weeks to establish the biofilter in marine aquaria compared to 4–5 weeks in freshwater)
Salinity (reactions occur more slowly at higher salinities, hence the reason it takes 8 weeks to establish the biofilter in marine aquaria compared to 4–5 weeks in freshwater)
Furthermore, the numbers and types of bacteria that live in the biofilter vary depending on pH, temperature, and salinity. An established, well-balanced aquarium should have zero ammonia and nitrite. If ammonia and/or nitrite are allowed to build up considerably, fish deaths result - a condition called "new tank syndrome."
Some chemicals used in the treatment of fish diseases may harm the biofilter. They include most antibiotics, copper sulfate, methylene blue, and trichlorfon. It is advisable to monitor levels of ammonia and nitrite following treatment, until the biofilter is re-established.
Biofilter requirements vary depending on species, water temperature, pH, fish size, stocking density, oxygen consumption, ammonia production, substrate type and surface area to volume ratio, filter efficiency, clogging by solids, water flow rate, and feed amount, frequency, and type.
Temperature
Fish are particularly sensitive to temperature changes, because they are poikilotherms. Water temperature affects their metabolism (metabolic rate), feed intake, growth, reproduction, physiological processes (affects the function of enzymes), disease immunity, and general activity.
Different species have specific temperature requirements within which they can live normally. The optimal and tolerance ranges for water temperature are specific to each species. For example, tropical fish are best kept at 24–28°C. For coldwater fish, such as goldfish and koi, their optimal water temperature is 20–24°C; however, they have wide tolerance ranges (1–35°C), allowing them to cope with the seasons. Water temperature can be maintained by the use of thermostatically controlled, submersible heaters and/or chillers, depending on whether the water needs to be warmed or cooled.
pH
The pH of the water is a measurement of its acidity, where values above 7.0 are alkaline and values below this are acidic. Numerous biological processes depend on pH, and there are species differences for pH requirements.
Fish held in environments that are too acidic have difficulty maintaining physiological functions, and the disturbances in ionic and osmotic balance can lead to haemoconcentration, increased blood pressure, and suppressed metabolic rate. Clinical signs displayed by koi kept in water at a pH of 4.5, for example, include excess mucus production, piping, flashing, fin twitching, inappetence, listlessness, jumping, loss of balance and buoyancy control, and death.
The pH of the water can affect fish through its effect on other chemical parameters. For example, low pH can result in the solubilisation of potentially toxic metals from sediments, while at high pH the toxic form of ammonia becomes more prevalent. Remember also that biofilter activity is inhibited at pH levels below 5.0.
When testing water for pH, it is important to remember that there may be diurnal fluctuations. This is mainly due to the consumption and production of carbon dioxide (CO2) by aquatic plants (including algae), and it is represented by this chemical equation:
H2O + CO2 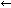
 H2CO3
H2CO3 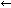
 H+ + HCO3-
H+ + HCO3-
Because of a net increase in CO2 overnight, the forward reaction is favoured, and so the water is most acidic in the morning. Conversely, because of the consumption of CO2 by plants/algae during the day, the reverse reaction is favoured, and so the water becomes more alkaline. These fluctuations can be minimised if there is sufficient buffering capacity of the water (see section on "carbonate hardness").
Poor tank hygiene (accumulation of organic material) is often the cause of low pH. In such cases, water changes with the removal of organic matter, and the addition of sodium bicarbonate (NaHCO3, baking soda), limestone, crushed coral or shells is recommended. Increases in pH should be made gradually, with no more than 0.5 units per day.
On the contrary, if the pH is excessively high, it can be lowered by the addition of phosphate salts (e.g., sodium phosphate monobasic and sodium phosphate dibasic) or acids like sulfuric and nitric. Note that phosphates are avoided if possible, since it can contribute to algal blooms. Peat filtration is a natural method used by aquarists.
The pH of the water can also influence the type or dose rate of medicines used to treat fish. Certain chemicals like Chloramine-T are more toxic in acidic pH and so a lower dose is needed; whereas chemicals like organophosphates degrade more quickly in alkaline conditions, and so higher dose rates or an alternative drug is advisable.
Ammonia
Ammonia (NH3) is a major waste product of fish. It is a strong cell poison and can cause damage to the gills, impairing gas exchange, and neurological damage. Clinical signs of ammonia toxicosis include (but are not limited to) increased mucus production, red or bleeding gills, darkening of body colouration, "gasping" for air at the water surface, and increased respiration rate. Gills from ammonia-treated fish will display severe histological and ultrastructural alterations including hyperplasia, hypertrophy and fusion of secondary lamellae, aneurysms, and presence of pleomorphic altered cells.
In water, it can be present in two forms: highly soluble toxic un-ionised ammonia (NH3, also known as free ammonia nitrogen or FAN), or the less toxic ammonium ion (NH4+) - the sum of which is known as TAN (total ammonia nitrogen). The proportion of toxic ammonia (FAN) is influenced by several factors; it is higher with increasing pH and temperature and with decreasing salinity. Of these, the pH of water is the most crucial factor. Water test kits usually measure the TAN. You can determine whether toxic levels of FAN are present with the help of a chart. Fish kills have been recorded at levels of 0.2–1.0 mg/L of FAN.
The most common causes of ammonia spikes include:
 Increased feeding rate (e.g., in the spring when fish are fed increasing amounts of food, and the biofilter has not had the chance to adapt to the new biological load)
Increased feeding rate (e.g., in the spring when fish are fed increasing amounts of food, and the biofilter has not had the chance to adapt to the new biological load)
 Increased stocking density (e.g., a large shipment of stock arrives at the fish shop, and the biofilter has not had time to adapt to the sudden increased biological load)
Increased stocking density (e.g., a large shipment of stock arrives at the fish shop, and the biofilter has not had time to adapt to the sudden increased biological load)
 Damage to biofilter (pump has stopped for a significant length of time, filter becomes clogged, filter is washed too thoroughly, chemicals including antibiotics used)
Damage to biofilter (pump has stopped for a significant length of time, filter becomes clogged, filter is washed too thoroughly, chemicals including antibiotics used)
Treatment for ammonia toxicosis involves performing multiple partial water changes (25–50% each time), using chemical filtration (e.g., zeolite in freshwater systems), and lowering the pH toward 6.0–6.5 (provided it stays within the tolerance level of its inhabitants). Chemical treatments to counteract ammonia toxicity are available commercially, and these contain formaldehyde that chemically binds ammonia to form methenamine (a safer compound which still relies on the biofilter to degrade). Clients can also supplement the biofilter with more nitrifying bacteria, which are available from fish shops. Feeding should be minimised and only gradually reintroduced.
Nitrite
Nitrite (NO2-) is generated from the oxidation of ammonia by nitrifying bacteria. Elevated levels often occur during the early stages of setting up new aquariums as the biofilter undergoes the nitrogen cycle process. A sudden spike in the nitrite usually means there is an imbalance in the system.
Nitrite poses a risk at levels above 0.5 mg/L, and it can be lethal when it exceeds 1.6 mg/L. Nitrite uptake by fish is via the chloride cells in the gills, and it causes the formation of methaemoglobin in the blood, blocking oxygen transport by the erythrocytes (similar to nitrate and carbon monoxide poisoning in mammals). Clinically, fish may be inappetent, lie on the bottom, and lose equilibrium. The gills of affected fish take on a dull, chocolate-brown colouration. Microscopic examination of blood smears may reveal intracytoplasmic vacuolation of erythrocytes and a regenerative anaemia. Fish suffering from nitrite toxicosis should not be handled if possible, because they can die from the increased stress.
The reasons for nitrite spikes are similar to those for ammonia. They could stem from something as simple as washing the biological filter media too thoroughly (Nitrobacter are not as adherent and may be washed away). This means that the ammonia can continue to be converted to nitrite, but the rate of conversion of nitrite to nitrate will be much reduced, and hence the nitrite spikes.
The relationship between total nitrite and un-ionised forms such as nitrous acid (HNO2) is inversely pH dependent. Both nitrite and nitrous acid are toxic to fish, but nitrous acid is more harmful. This explains why nitrite is more toxic in acidic water. It is also more toxic in soft water, at higher temperatures, and in lower salinity.
Nitrite toxicosis is addressed by withdrawing food, followed by multiple, large, partial water changes (25–50%). Methylene blue should be added at a rate of 1–2 mg/L to reverse the process of methaemoglobin formation (predilute the chemical and avoid the biofilter intake). Salinity (NaCl) should be increased by up to 5 mg/L (and within the tolerance ranges of its inhabitants) to competitively inhibit nitrite uptake by the fish. The pH of the water can be raised to the highest end of the fish's tolerance range, and the water temperature reduced, to minimise the proportion of the more toxic form of nitrite. Provide supplemental aeration, and additional nitrifying bacteria for the biofilter. Fish should return to normal within 24 hours, although mortalities can still be expected up to 2 weeks later.
Nitrate
Nitrate is the end-product of organic and inorganic decay, and it accumulates in tanks over time (high levels indicate poor husbandry), especially those without plants. Nitrate level should be kept below 50 mg/L. At levels >200–400 mg/L, it creates considerable stress to fish, retards their growth rate, lowers their capacity to resist disease, and delays wound healing. Reports suggest that high nitrates can be toxic under conditions of reduced dissolved oxygen, because it can damage fish erythrocytes.
Carbonate Hardness (KH) or Alkalinity
This is the measurement of the capacity for water to neutralise an acid (i.e., the buffering capacity against pH crashes). Most aquarium KH test kits measure total alkalinity (or total pH buffering capacity). The alkalinity is primarily composed of bicarbonate ions (HCO3-) and carbonate ions (CO3-2), hence the common name of "KH". However, it may also consist of diphosphates (H2PO4-), sulfates, and borates. KH is essential to stabilise the pH of water; it is an important source of energy for nitrifying bacteria; and it is used by plants for photosynthesis (when carbon dioxide is absent).
Most aquarium test kits measure "total alkalinity" rather than just the carbonates. Generally, the desired KH for the majority of freshwater fish is 60–80 mg/L; for Rift Lake cichlid it is 120–200 mg/L; and 120–200 mg/L for marine. Levels >800 mg/L have been implicated in fish kills.
Alkalinity can be increased by the addition of salts of carbonates, hydroxides, and the speed and stability of effect are influenced by their solubility.
General Hardness (GH)
This is a measurement of all chemically bivalent cations (primarily comprising calcium and magnesium) that in nature are related to the geology of the water source. The units of measurement can be expressed in several ways, where: 1 dH = 17.8 mg/L = 17.8 ppm = 0.35 mEq/L.
Typically, water is described as 'hard' if the GH is >300 mg/L, and 'soft' if it is <150 mg/L. Hard water is noticeable because it causes scaling problems with plumbing/pipework and fittings. The fish's requirements for the level of general hardness depends on the species and their native origins.
For some fish, their major source of calcium and magnesium is from the water, so the GH is important for skeleton and scale growth. Shelled organisms like crustacea and molluscs also use these ions. Excessively high GH will result in zinc deficiency cataracts and nephrocalcinosis. Hard water can also chelate drugs like the tetracyclines, rendering them ineffective when administered in water or orally. Thus, higher dose rates or alternative drugs will need to be considered.
Water can be softened by the use of distilled water, rainwater, reverse osmosis water, or peat. Calcium and magnesium salts can be added to water to raise the GH to the required levels, depending on the fish species.
Salinity
Salinity is the measurement of total soluble salts contained within a volume of water, and depending on the means of measurement, it can be expressed in several ways and they are tabulated below.
Table 1. Classification of water salinity expressed in different ways
|
Common term
|
Scientific terminology
|
Salinity (ppt) or (g/L)
|
Conductivity (mS/m)*
|
TDS (mg/L)
|
Specific gravity (SG)
|
|
Sea water
|
Euryhaline
|
30–40
|
5,655–7,540
|
>30,250
|
1.024–1.030
|
|
Brackish water
|
Mesohaline
|
10–18
|
1,885–3,393
|
495–1,485
|
1.018
|
|
Freshwater
|
Freshwater
|
<0.21
|
5–39.6
|
<495
|
1.000
|
It is essential to maintain the correct salinity in an aquarium (marine) because of the fine balance between the concentration of salts in a fish's body compared with that of the surrounding water. The ability of fish to control small fluctuations in these concentrations is called osmoregulation. Fish will experience osmotic stress if the salinity is not within the natural ranges. Death can result if fish are exposed to salinities outside their tolerance ranges for prolonged periods.
The most common occurrence of salinity variation is the gradual increase in salinity due to evaporation. This can occur particularly when reduced water levels in aquaria are simply topped up without a water change. This is a good tool to check on the adequacy of husbandry.
Salinity is commonly modified when treating sick fish, where it is altered by 0–5 g/L to reduce osmoregulatory stress (salinity is increased in freshwater fish, and it is reduced in marines). Suitable types of salt used include rock salt, sea salt, and pool salt. Do not use table salt that is iodised or those that may contain anti-caking agents.
Oxygen
Oxygen is vital for a healthy aquarium. The maximum oxygen-carrying capacity of water is affected by the temperature and salinity, where dissolved gases are reduced at higher temperatures and in higher salinity water. Healthy systems should be 70–100% saturated, and as a general guide, fish need >5 mg/L of dissolved oxygen.
Oxygen saturation of >100% has been implicated in gas bubble disease, and dissolved oxygen of <3 mg/L has been associated with fish kills. In cases of low dissolved oxygen (DO), fish become listless and they gather at the water surface or at water inlets.
Additional oxygenation may be required when there is high stocking density, large fish, low water movement, high feeding rate, high metabolic rate, high water temperature, algal blooms, bacterial blooms, excessive planting, and during certain chemical treatments (e.g., formalin, salt bath).
In cases of emergency, oxygen may be increased by dosing with 3% hydrogen peroxide at 0.25 ml/L (it decomposes to yield oxygen and water).
Chlorine/Chloramine
Chlorine (Cl2) is commonly found in tap water at a rate of 0.5–2.0 mg/L. There are large species variations in terms of tolerance; however, adverse effects on fish can occur at levels as little as 0.02 mg/L. It can be lethal to fish at levels above 0.04–0.10 mg/L. Chlorine exposure causes oxidative damage to erythrocytes and necrosis in the gills, liver, and spleen. Chlorine is also more toxic at a lower pH.
Testing for chlorine level is not always possible or necessary, because a diagnosis can often be made from the clinical history. Treatment involves the addition of 7.4 mg/L sodium thiosulphate (chlorine neutraliser) to every 1 mg/L chlorine that is present in the water. Since the neutralising reaction consumes oxygen and lowers the pH, vigorous aeration and buffering with sodium bicarbonate is recommended.
References
1. Loh R. Fish Vetting Essentials. Perth, Australia: Richmond Loh Publishing; 2011.