Stephen J. Divers, BVetMed, DZooMed, DACZM, DECZM(herpetology), FRCVS, Department of Small Animal Medicine and Surgery (Zoological Medicine)
Abstract
Blood pressure has long been advocated as an important cardiovascular parameter to measure and maintain in anesthetized patients because it represents quantification of blood flow and tissue perfusion.4 Despite the inherent value of measuring blood pressure, such methods are rarely used in reptile anesthesiology.1,7,8 Blood pressure is typically under close autonomic regulation, is largely mediated by the baroreflex, and can be quantified by systolic arterial pressure (SAP), diastolic arterial pressure (DAP) and mean arterial pressure (MAP).5 Despite previous recommendations by Lichtenberger,6 other authors have considered indirect blood pressure monitoring to be inaccurate and imprecise in reptiles.1,3,6 In green iguanas (Iguana iguana) indirect measurements suffered an 81% failure rate, while in boid snakes indirect measurements frequently over-estimated SAP, under-estimated DAP and MAP, and at SAP<100 mm Hg all three pressure variables were variably under-estimated.2,3 Conversely, direct measurements of blood pressure in iguanids and boids have been found to be accurate and repeatable1,2; however, the need for surgical exposure and catheterization make such techniques impractical for most clinical work (Figures 1 and 2). Isoflurane has been shown to have profound effects on iguanid blood pressures, with isoflurane at 3% decreasing MAP from 65-85 to <40 mm Hg.1 Despite previous comments that atropine has little effect in reptiles, atropine does significantly increase heart rate while maintaining MAP, while b1-antagonists like atenolol reduce heart rate, again while maintaining MAP.5 Medetomidine, atipamezole, dopamine and phrenylephrine appear to have little to no effect in green iguanas; however, norepinephrine at 0.4 and 0.5 µg/kg/min significantly increased MAP from 27 to 66 mm Hg (unpublished data).
Figure 1. Surgical placement of a telemetry blood pressure monitoring device in a green iguana

Left – placing a stay suture around the distal carotid; Middle – placing a vasculature pic through a nick in the arterial wall to facilitate the insertion of the arterial catheter; Right – Subcutaneous placement of the telemetry end of the catheter to permit hand-free blood pressure monitoring.
Figure 2. Continuous direct blood pressure reading from a conscious green iguana using an implanted telemetry arterial catheter
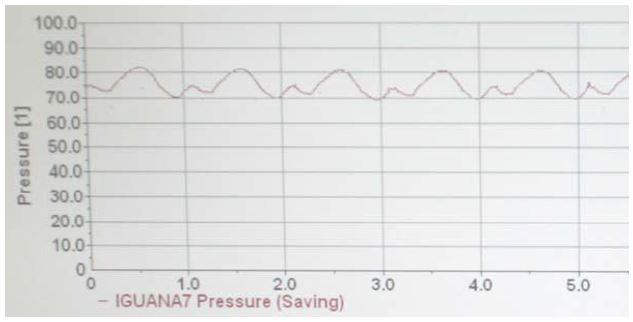
In this case, the animal has a heart rate of 60 beats/min, with a SAP of 81 mm Hg and DAP of 70 mm Hg.
Arterial blood gases are used to assess the adequacy of ventilation (PaCO2), blood oxygenation (PaO2), and acid-base status (pH, PaCO2). There are a variety of portable units in practice but most only directly measures pH, PCO2, and PO2 with lactate, bicarbonate, TCO2, BE, and SaO2 calculated using human algorithms. Unsurprisingly, some controversy and unique problems exist regarding the interpretation of blood gases in reptiles. Some argue that all reptile samples should be corrected to 37°C which results in decreases in values for pH, CO2, and PO2. Others have advocated correcting to the reptile’s body temperature which would result in higher values for these same parameters. Carotid collection is preferred because it accurately reflects blood flow to brain. Intracardiac sampling is likely to result in a mixed arterial-venous sample. Venous samples can only indirectly reflect PaCO2 and ventilation, and are even less likely to accurately reflect PaO2. Furthermore, venous PCO2 can be elevated due to increased metabolism or impaired tissue perfusion. A recent study in green iguanas has indicated that there are significant circadian changes in arterial PO2 and SaO2, and that pulse oximetry (SpO2) is an inaccurate measure for SaO2 due to under-estimation (Table 1).5 There were no circadian effects observed from venous samples, but significant differences existed between arterial and venous results for PCO2, PO2, and SaO2/SvO2. There were no significant differences between arterial and venous results for lactate, bicarbonate, TCO2, and BE. To summarize, arterial samples are needed for PO2 and SaO2 evaluations, but venous samples are useful for other parameters incl pH, lactate, bicarbonate, TCO2, and BE.’’
Table 1. Mean ± SD arterial and venous blood gas values as determined at 37°C in 15 conscious green iguanas (Iguana iguana) breathing room air (Hernandez et al., 2011)a,b,c
|
Parameter
|
Arterial
9 to 11 am
|
3 to 5 pm
|
P valued
|
Venous
9 to 11 am
|
3 to 5 pm
|
P valued
|
|
pH
|
7.29±0.11
(7.38±0.12)
|
7.29±0.11
(7.38±0.12)
|
0.499
(0.500)
|
7.31e
(7.36±0.13)
|
7.25 e
(7.32±0.15)
|
0.594
(0.729)
|
|
PCO2 (mm Hg)
|
42±9
(32±7)
|
46±10
(35±8)
|
0.186
(0.187)
|
49 d
(36±7)
|
56 d
(42±11)
|
0.084
(0.056)
|
|
PO2 (mm Hg)
|
81±19†
(54±15)
|
94±21†
(64±16)
|
0.043
(0.044)
|
46±23
(30±15)
|
37±15
(24±9)
|
0.929
(0.878)
|
|
Lactate (mmol/L)
|
2.7±1.1
|
4.2±3.4
|
0.192
|
2.3 e
|
3.5 e
|
0.975
|
|
Bicarbonate (mmol/L)
|
20±4
|
22±4
|
0.111
|
22±5
|
24±4
|
0.128
|
|
TCO2 (mmol/L)
|
22±4
|
24±5
|
0.134
|
24±5
|
25±4
|
0.126
|
|
Base excess (mmol/L)
|
–6.3±5.5
|
–4.3±5.3
|
0.199
|
–5±6
|
–4±6
|
0.306
|
|
SaO2 (%)
|
92±6
|
95±3
|
0.027
|
84 e
|
49 e
|
0.064
|
|
SpO2 (%)f
|
86±6
|
ND
|
ND
|
ND
|
ND
|
ND
|
aArterial and venous blood samples were collected in the morning and afternoon to determine the effect of circadian rhythm on blood gas parameters.
bValues in parentheses are corrected for a body temperature of 30°C.
cData are reported as mean ± SD unless indicated otherwise.
dA value of P<0.05 was considered significant. eValues are reported as medians.
fOxygen saturation as measured by pulse oximetry.
Acknowledgments
Sincere thanks to Drs Chinnadurai, DeVoe, Koenig, Hernandez, Schumacher, Read, and Lewis for involving me in their research at the College of Veterinary Medicine, University of Georgia.
Literature Cited
1. Chinnadurai, S.K., R. DeVoe, A. Koenig, N. Gadsen, and S.J. Divers. 2010. Comparison of an implantable telemetry device and an oscillometric monitor for measurement of blood pressure in anaesthetized and unretrained green iguanas (Iguana iguana). Vet. Anaesth. Analg. accepted for publication.
2. Chinnadurai, S.K., S.J. Divers, R. DeVoe, N. Gadsen, and A. Koenig. 2010. Effects of multiple adrenergic agonists on blood pressure in green iguanas (Iguana iguana). Vet. Anaesth. Analg. accepted for publication.
3. Chinnadurai, S.K., A. Wrenn, and R.S. DeVoe. 2009. Evaluation of noninvasive oscillometric blood pressure monitoring in anesthetized boid snakes. J. Am. Vet. Med. Assoc. 234: 625–630.
4. Heard, D. 2007. Monitoring. In: G. West, D. Heard, and N. Caulkett (eds.). Zoo Animal and Wildlife Immobilization and Anesthesia., ed. Blackwell Publishing, Ames, Iowa. 91.
5. Hernandez, S.M., J. Schumacher, S.J. Lewis, A. Odoi, and S.J. Divers. 2011. Selected cardiopulmonary values and baroreceptor reflex in conscious green iguanas (Iguana iguana). Am. J. Vet. Res. 72: 1519–1526.
6. Lichtenberger, M. 2007. Emergency and critical care medicine. Vet. Clinics N. Am., Exotic Anim. Prac. 10: 275–677.
7. Read, M.R. 2004. Evaluation of the use of anesthesia and analgesia in reptiles. J. Am. Vet. Med. Assoc. 224: 547–552.
8. Schumacher, J., and T. Yelen. 2006. Anesthesia and analgesia. In: D.R. Mader (ed.). Reptile Medicine and Surgery, 2nd ed. Elsevier, St. Louis, Missouri. 442–452.