Preliminary Report on Thyroid Hormone Levels in Tissues of Stranded Cetaceans
Introduction
Exposure to environmental pollutants is one of most important risks for marine ecosystem and species. Most important contaminants are those which persist for long time in the environment and which can concentrate in animal and lead to subtle but important effects, such as endocrine disruption.2,3
Being a closed basin, Mediterranean Sea can experience great risk from pollution. Thus knowing the actual levels of pollutant burden and pathophysiological consequences can let us better assess the risk for ecosystems, endangered populations and human health.
In monitoring activities focusing on endangered species like sea mammals and turtles, stranded dead animals are often used, as they are easier to collect with respect to live, free ranging animals. Sampling from these individuals only rarely allows the collection of blood samples or the efficient evaluation of markers of effect, particularly those concerning thyroid activity. Setting a method for thyroid hormones quantification in tissues of stranded dead animals can allow at least the estimation of thyroid gland functionality, if not its complete evaluation; this will give some information on the endocrine status of the individuals at stranding.
The present study aims at the evaluation of the applicability of a thyroid hormone extraction method developed for fish tissues to cetacean tissues, in order to create a new tool for the evaluation of endocrine disruption effects of pollutants in highly protected species and/or endangered local populations.
Materials And Methods
Samples from stranded animals collected along Italian Northern Adriatic Sea and Israeli Mediterranean coasts were used. Tissue collection was conditioned by the conservation status of carcasses and by the decomposition level of tissues themselves. A total of nine liver and muscle, six kidney, five blubber, four melon, two heart and lung, one ventral muscle and skin samples were collected from 10 bottlenose dolphins and one sperm whale in Italy; eight kidney, 10 muscle and blubber and six liver samples from three Cuvier's beaked whales, one sperm whale, two Risso's dolphins, two stripped dolphins, two minke whales, one rough-toothed dolphin and one fin whale were obtained from Israel. Thyroid hormones extraction from tissues (0.3 g fresh weight) was performed following Crane et al1 and they were quantified by Radioimmune Assay with available commercial kits. Due to low number of samples, no statistical analysis could be performed, so that only a simple comparison of levels was done.
Results And Discussion
The applied extraction method proved to be efficient for T3 only, which was always above the limit of detection (LOD), while T4 levels were close to or below LOD. This may be expected because data concerning thyroid hormones concentration in animals' fish tissues indicate about low levels of thyroxine T4 in fish tissues and detectable levels of triiodothyronine T3.2.3 The fact that 14 of 73 samples presented T4 concentrations slightly higher than LOD seems to confirm this hypothesis and the applicability of the method to these types of tissues.
Mean values of T3 were higher in kidney of many species, followed by muscle and liver (i.e., 43.23 ng/g in stripped dolphin kidney, 21.84 ± 19.87 ng/g in Risso's dolphin muscle, 12.93 ± 8.45 ng/g in minke whale liver). This makes it possible to consider kidney as marker tissue for T3 quantification. These findings are somewhat unexpected, as metabolism and degradation of thyroid hormones occurs in liver, which could have been considered a storage tissue, presenting highest hormone concentrations. Instead, kidney and muscle, which are usually among the less degraded tissues in dead stranded animals, seems to be the best markers of thyroid functioning. To confirm this hypothesis, additional samples need to be analyzed.
No statistical difference was observed between species and tissue considered; however a sampling bias should be considered, linked to the large differences in species and tissues numbers.
| Figure 1. | 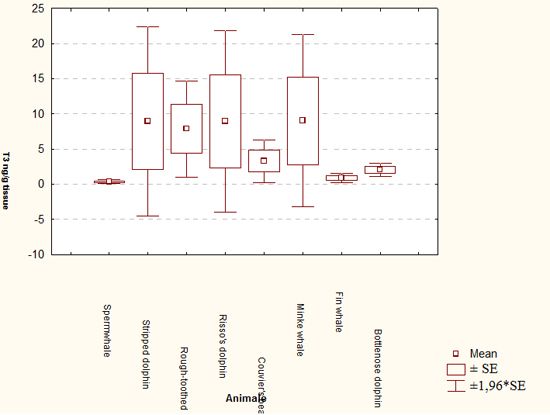
Mean T3 levels (ng/g tissue) in different species considered (all tissues combined). |
|
| |
No other information is available on thyroid hormones tissue concentration in cetaceans or other mammals. Scarce data are available concerning blood levels: Orlov et al4 reported mean T3 concentrations of 1.27 ± 0.04 nmol/l (0.,0195 ± 0.,0006 ng/dl) in bottlenose dolphin, and St Aubin and Geraci5 reported mean hormone levels in beluga of 2.7 ± 0.7 nmol/l (0.04158 ± 0.,010 ng/dl).
To our knowledge, the present data are to be considered as the first report concerning thyroid hormone levels in tissues of cetaceans. Further development of an applied analytical method and its application to a larger number of samples, possibly including tissues from healthy, controlled animals (i.e., those housed in marine parks) can introduce a new tool for ecotoxicological risk assessment, especially to protect highly endangered species. Indeed, the definition of establishing correlation between plasma and tissues levels of hormones will allow the assessment of thyroid activity in dead animals, expanding the range of possible adverse effects induced by pollutants which can be evaluated in these endangered species, thus improving the protection activities.
Acknowledgements
The authors whish to thank Prof. Dami Kerem, of Israel Marine Mammals Research and Assistance Centre, Haifa, Israel, for giving us part of the samples.
References
1. Crane HM, Pickford DB, Hutchinson TH, Brown JA. 2004. Developmental changes of thyroid hormones in the fathead minnow, Pimephales promelas. Gen. Comp. Endocrinol., 139(1): 55-60.
2. Keen PL, Higgs DA, Hall KJ, Ikonomou M. 2005. Effects of dietary exposure of 4-nonylphenol on growth and smoltification of juvenile coho salmon (Oncorhynchus kisutch). Sci. Tot. Environ. 349:81-94.
3. McCormick SD, O'Dea MF, Moeckel AM, Lerner DT, Bjornsson BT. 2005. Endocrine disruption of parr-smolt transformation and seawater tolerance of Atlantic salmon by 4-nonylphenol and 17[beta]-estradiol. Gen. Comp. Endocrinol. 142:280-288.
4. Orlov MM, Mukhlia AM, Kulikov NA. 1988. Hormonal indices of the normal dolphin Tursiops truncatus and in the dynamics of experimental stress. Zh. Evol. Biokhim. Fiziol., 24: 557-563.
5. St Aubin DJ, Geraci JR. 1992. Thyroid hormone balance in beluga whales, Delphinapterus leucas: dynamics after capture and influence of thyrotropin. Can. J. Vet. Res., 56: 1-5.