Primary brain tumors are a significant cause of morbidity and mortality in small animal companion animals, particularly in dogs. With more widespread availability of advanced imaging techniques, the number of animals being diagnosed with intracranial mass lesions has increased significantly. The most commonly diagnosed tumors are meningioma, followed by glial tumors (astrocytoma, oligodendroglioma) and choroid plexus tumors. Information relating to imaging characteristics of specific tumor types has been published, however imaging can never provide a specific diagnosis. As with neoplastic disease affecting any other organ system in the body, definitive diagnosis has been historically confirmed primarily based on the results of biopsy, histopathology and immunohistochemistry.
Biopsy of primary brain tumors presents a number of location specific problems, primarily involving the relative inaccessibility of lesions, together with the significant risks associated with surgical biopsy in many cases. Although limited in availability at this time, recent advances in the development of stereotactic CT guided biopsy of tumors has done much to improve the likelihood of obtaining an accurate ante mortem diagnosis, allowing for a more appropriate and informed approach to therapeutic planning.
In our experience, it is not uncommon for lesions to be diagnosed by biopsy as something very different than the suspected diagnosis based on MRI. In some instances even the category of disease may even change for example inflammatory disease being misdiagnosed as neoplastic disease. This can have dramatic consequences on appropriate therapeutic planning, when the expense and potential adverse effects of treatments such as radiation therapy, or surgery are considered.
Click on an image to see a larger view.
| CT guided stereotactic brain biopsy. | 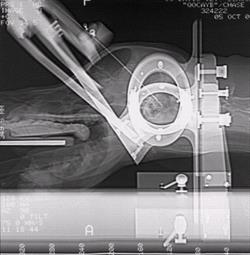
|
|
| |
| |
Conventional Therapy
In general, conventional therapeutic approaches to brain tumors in people, and animals have involved a combination of surgical debulking/resection, chemotherapy, and radiation therapy. A large body of clinical data exists in human medicine pertaining to the relative efficacy of these therapies for specific tumors, together with the expected prognosis. Very little similar objective information is available for the dog, even relating to the normal progression of brain tumors in the absence of treatment. Small case study series, lack of ante mortem (or post mortem) diagnoses, differing treatment plans, the high degree of variability associated with an end point often defined by euthanasia, and variation in clinical severity at presentation have made the comparison of canine and human data very difficult.
In general, the majority of meningiomas in humans are treated surgically with a mortality rate of less than 10%. Recurring tumors (and aggressive tumors) may be treated with radiation and repeat surgical resection. Human gliomas, grades I-II, II and IV are generally treated with surgical resection followed by radiation and chemotherapy, particularly with high grade tumors. The prognosis for these tumors varies from a 15 year survival rate of ~ 15% for Grade I, II tumors to a median survival of 4-16 months for high grade tumors. In fact, despite improvements in surgical techniques, radiation therapy (including radio surgery) and new chemotherapeutic agents, the prognosis for human patients with high grade gliomas has not altered significantly over the last 20 years!
With the exception of meningiomas in cats, and sometimes in dogs, the prognosis for the majority of primary brain tumors (particularly intra axial tumors) even with conventional treatments is guarded to poor. Survival with symptomatic treatment alone is often measured in terms of weeks in most cases. Meningiomas treated with surgery +/- radiation or radiation alone may have a survival in the region of 6m-2 years in dogs. Survival times are often significantly less for intra axial tumors with all treatment options. However good quality of life for significant periods of time can be achieved with many patients in selected cases where neurological signs are not too severe, tumors are located in areas amenable to surgical resection, and/or animals respond well to palliative therapy to reduce peritumoral edema (corticosteroids) and control symptomatic seizures.
Advances in Conventional Therapy
Improved pre surgical imaging capabilities, together with improved surgical techniques and equipment are likely to lead to some modest improvements in prognosis, particularly for readily accessible extra axial tumors.
Conventional chemotherapy has advanced very little in both the human and veterinary fields in the last 2 decades. Use of adjunctive chemotherapy such as hydroxyurea in the treatment of meningioma, either following surgery or following recurrence post surgery may be beneficial, however no objective data are available at this time. The only "new" chemotherapeutic agent to be approved for the treatment of human glioma in the last 2 decades is the alkylating agent temozolamide (Temodar), however clinical gains are small. It is unclear whether Temodar offers any significant advantages over standard (much cheaper!) alkylating agents such as CCNU in dogs with gliomas.
Although little data are available to make specific conclusions, radiation therapy is generally accepted to be the most useful adjunctive or sole therapy (where surgery is not possible), particularly for intraaxial tumors. Standard radiation treatment involves 15-20 fractionated doses of radiation over a 3-4 week treatment course, and significant expense. Advances in the ability to deliver radiation to tumors while sparing normal brain (e.g., intensity modulated radiation therapy IMRT) are likely to result in improved survival, and are becoming available at a limited number of veterinary institutions. More advanced stereotactic radiosurgical techniques such as the Gamma Knife and LINAC knife deliver very high doses of radiation to tumors in a single treatment while sparing normal tissues. These facilities are available to veterinarians at only a small number of research facilities, however this is likely to change in the next several years. Radiation involving a single treatment, and therefore a single anesthesia, has many potential benefits for veterinary patients. Preliminary studies in dogs with brain tumors suggested that single dose radiosurgery can be as effective as standard fractionated radiation treatment in selected tumors.
Molecular Diagnostics and Targeted Therapy
Over the past 15-20 years, there has been a large effort to understand the specific molecular abnormalities underlying the development and progression of human primary brain tumors. Many of these abnormalities involve tumor suppressor genes, oncogenes and pathways involved in cell cycle regulation and angiogenesis. This has had an impact in two major ways.
1. By defining tumors in terms of their molecular characteristics, it has been possible to further classify apparently histologically identical tumors into separate groups. This has had a major impact on the ability to predict prognosis and response to conventional therapies. For example:
Many oligodendroglial tumors exhibit loss of chromosomes 1p and 19q. Loss of 1p or combined loss of 1p and 19q is associated with increased chemosensitivity and increased survival. Over expression of the epidermal growth factor receptor (EGFR) insulin like growth factor receptor (IGF1R) in gliomas is associated with radio resistance; similarly, over expression of the vascular endothelial growth factor (VEGF) and its receptors (VEGFR) is associated with a poor prognosis.
The ability to predict response to treatment based on the presence or absence of specific molecular markers has taken clinical pathology/histology to a new level and not only helps to select appropriate patients for specific treatments, but also helps to more realistically assess efficacy of therapeutic regimens which may have appeared ineffective when applied to a "mixed" population of uncharacterized and potentially inappropriate tumors.
2. Because of the relatively poor response of many primary brain tumors to conventional therapies, many novel approaches have been designed. Many of these approaches target the molecular abnormalities known to be present in specific tumors such as replacing abnormal or absent tumor suppressor gene function (e.g., TP53), or inhibiting growth factors known to be important in angiogenesis or tumor growth (e.g., VEGF, EGF). If appropriate pathways are present, such targeted treatments can be extremely effective, as has been shown in the remarkable success of ST1571 ("Gleevac") in the treatment of chronic myeloid leukemia. (ST1571 targets the constitutively activated BCR-ABL tyrosine kinase receptor) Many similar treatments are currently in development and clinical trials in brain tumor patients. Additionally, over expression of markers specific to brain tumors can be used to target non specific therapeutics such as toxins or more conventional chemotherapeutic agents. Gene therapy using viral vectors such as adenovirus, retrovirus and adeno-associated virus has also been assessed in both experimental and clinical tumors. The ability of many viruses to transduce tumor cells (or normal brain) depends on many factors including appropriate cell surface targets. Generation of promoter specific viral constructs also adds an additional targeting step helping ensure that therapeutic gene expression occurs only in the appropriate cell types.
There is little published data documenting the molecular characteristics of canine brain tumors, however several research groups are currently involved in work in several areas including expression and altered regulation of growth factor pathways; tumor suppressor gene function; telomerase activity, gene array expression profiling and chromosomal alterations. The recent release of the canine genome data will help enormously in promoting this basic research and help ensure that the veterinary profession is able to benefit from current and future advances in human brain tumor therapy, as well as potentially playing an integral part in both basic and applied clinical research.
Delivery of Therapeutic Agents
A wide variety of methods have been employed to deliver therapeutic agents to brain tumors. Many strategies have involved systemic delivery of agents either orally or intravenously. Some drugs (e.g., standard chemotherapeutic agents) are relatively non specific with respect to their potential targets, whereas others (e.g., small molecule tyrosine kinase inhibitors such as Gleevac) may have a more precisely defined target despite the systemic delivery. Even with the use of targeted therapies, the likelihood of significant systemic side effects is a major concern with drugs delivered in this manner. Ability of drugs to cross the blood brain barrier is also a factor that can significantly limit the efficacy of systemically delivered therapies, and many factors such as molecular weight, permeability of vasculature, drug stability and diffusion characteristics as well as tumor related factors are critical to attain effective cellular levels of anti tumor drugs. Local "targeted" delivery of therapies directly into tumor tissue has been advocated as a way to increase both the efficacy of many therapeutic agents whilst at the same time decreasing the likelihood of significant systemic toxicity. Therapeutic agents may be delivered directly at surgery following excision/debulking of tumors, or by stereotactic injection. Recent advances in injection of agents by convection enhanced delivery (CED) (over several hours), have shown great promise, and may allow highly accurate and comprehensive delivery of therapeutics to a defined area of tumor and/or surrounding brain.
Preliminary results with CED using a novel chemotherapeutic agent CPT-11 (topoisomerase I inhibitor) in an ongoing clinical trial in dogs with spontaneous gliomas are encouraging, and demonstrate the feasibility of targeted delivery in veterinary patients.
Click on the image to see a larger view.
| CED of CPT-11 chemotherapy in a canine glioma. | 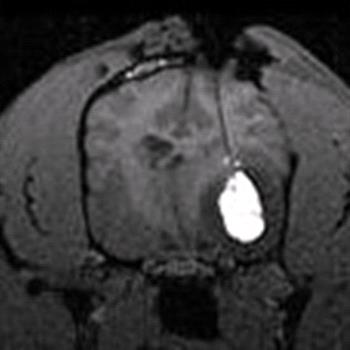
|
|
| |