Overview of Neonatal Management Techniques at the U.S. Navy Marine Mammal Program
Abstract
Background
In domestic animals, the ability to safely examine and obtain biologic samples from the neonate has been essential to successful reproductive programs. Only recently have handling techniques been developed for bottlenose dolphin neonates and calves to allow for safe examination and diagnostic sampling. The U.S. Navy Marine Mammal Program (NMMP) at Spawar Systems Center San Diego has employed new techniques in a proactive preventive medicine program for its most recent set of calves, which has facilitated blood sampling, morphometric data collection, ultrasonography, and radiography as early as 12 hours postpartum. The veterinary care aspect of a reproductive program is not complete without addressing the daily nutritional requirements and behavioral development of the calf. Young orphaned calves have been successfully supported with bottle and tube feeding, however this approach can become labor intensive and does not specifically address the establishment of a mother-calf bond. The exploration and utilization of dolphin alloparenting has provided our program with a solution that meets the nutritional and social needs of orphan calves while requiring little human intervention. The ultimate goal of this work was to increase our understanding of methods to improve calf survivability and establish safe calf handling and diagnostic techniques that can be utilized industry wide.
Neonatal Handling
Our historical data suggest that method of restraint for a bottlenose dolphin calf may be the single most important factor in providing successful veterinary care to these animals. For neonates in particular, "in water" procedures are tolerated far better than those performed "out of water." It appears that if neonates are allowed to swim or undulate and remain in acoustic contact with the mother, we can avoid the shock-like response we have seen when they have been completely removed from their aquatic environment. While vital signs are always monitored, these numbers can vary widely depending on the age of the neonate and number of times previously restrained. Duration of restraint for neonates and young calves has ranged from 2-30 minutes, the latter allowing for thorough diagnostic workups.
Preventive Medicine
Our neonatal preventive medicine program in 2005 included 1) behavioral monitoring, 2) respiratory rate monitoring, 3) blood sampling, and 4) morphometric data collection. Behavioral monitoring was achieved by using handheld devices to collect continuous data on specific calf and maternal behaviors (see Figure 1). Calf behaviors included nursing, swimming position, vocalizations, and independence. Maternal behaviors included calf discipline, calf retrieving/herding, calf neglect, and nursing-associated behaviors. From these data, nursing behavior was assessed and nursing intervals were calculated. Handheld devices were also used to monitor dam and calf respiratory rates, providing data that could be quickly analyzed and proving to be a valuable indicator of general health. Calf respiratory rates ranged from 10-30 breaths per 5 minutes in the first 24 hours of life, and ranged from 10-36 breaths per 5 minutes in the first 3 months of life (higher rates noted with increased levels of independence). It is important to note that neonatal respiration rates mirrored the dam's respiration rate immediately postpartum. More data is needed to determine how long this stage lasts. Blood was sampled for routine CBC and chemistry analyses. Table 1 summarizes our CBC and chemistry data from neonates and calves that have lived to be greater than one-year-old. Please note that our sample size is small so should only be used as a point of reference. The acquisition of blood samples allowed us to begin investigations into the role of passive transfer by measuring serum IgG levels, which are summarized in the Ruiz et al. abstract (1).
Alloparenting
Alloparenting and more specifically allomaternal care is a well-documented phenomenon in both domestic and wild animals, including bottlenose dolphins (2-5). Alloparenting is best defined as behavior carried out by a non-parent that appears parental in nature and benefits the younger animal. This behavior appears strong in bottlenose dolphins and has allowed us to pursue alloparenting as a means to rear calves. By utilizing either induced or spontaneous lactation and post-weaning lactation periods, the NMMP breeding program has staged lactating females to be available at the time of each parturition. For females identified as good candidates for alloparenting, a healthy status is confirmed and behavioral preparations for weaning take place prior to the upcoming parturition. If alloparenting becomes necessary, weaning takes place and the new alloparent-calf relationship established. In our experience, this bond may not be immediate and we have learned to be patient as nursing and mothering behavior develops.
Case Report
On 10 September 2005, a female calf was born in the ocean enclosures of the NMMP Breeding Program. This was the sixth calf born to a female that had historically displayed no evidence of nursing behavior to four previous live calves (one spontaneous abortion). Based on data collected at our facility, neonates that have not nursed by 12 hours post-partum had a poor chance of survival. The decision was made to allow up to 10 hours for this female to display successful nursing behavior. Successful nursing did not occur by hour 10, and both the mother and calf were pulled to facilitate the collection of colostrum from the dam, oral administration of fresh colostrum to the calf, health assessment of the calf including blood collection, and introduction of the calf to a lactating female. The process of handling the calf for the oral administration of colostrum occurred a total of seven times over a sixteen hour period (12 to 28 hrs post-partum) with the calf receiving between 12-33 cc of colostrum each session with a total volume of 180 cc being administered. During this same time period, the calf was nursing off of the allomother. First nursing attempt occurred within one hour of introduction. Calf monitoring during restraint revealed heart rates ranging between 125-156 beats per minute and respiratory rates ranging between 5-17 breaths per minute.
During the first day of life, the calf sustained a third degree abrasion to its rostrum. At day 10, the wound showed signs of infection and blood work revealed an inflammatory hemogram. The wound was treated topically and systemic antibiotics were administered (ceftriaxone 10mg/kg IM SID). The wound healed slowly but without complication. Since we were unsure of the success of our colostrum administration and protective ability of the calf's immune system, serial serum samples drawn were sent to the University of Florida College of Veterinary Medicine for serum IgG levels (1). The IgG was undetectable until day 12 and increased to a value of 1.25 mg/ml by day 25 (in-house adult bottlenose dolphin range = 1.8-4.0 mg/ml). In order to accurately assess these data, it is critical that we continue to collect samples from dolphin neonates over the first few weeks of life.
Secondary pneumonia is a common cause of death in dolphin calves with suspected partial or complete failure of passive transfer, so it was important to monitor pulmonary health. Thoracic ultrasound was performed routinely in the water. On day 88, ultrasound revealed evidence of low-grade pulmonary disease which coincided with an inflammatory hemogram. The trainers had achieved a consistent feeding schedule with the animal, so we were able to take advantage of an oral antibiotic regime (amoxicillin 10mg/kg PO BID for 16 days, from day 88-103 of life). Follow-up blood work and ultrasound revealed no significant response to therapy. Antibiotic selection was changed to cefaclor 10 mg/kg PO BID for 30 days, from day 103-132 of life. Follow-up blood work revealed response to therapy and resolution of inflammation. Before discontinuing antibiotics, a thoracic series of digital radiographs was obtained. To accomplish this, the animal was approximated to increasing time out of the water in a stretcher, and the radiographs were taken during a total "out of water" period of 12 minutes. Radiographs revealed no significant findings and antibiotics were discontinued. Special precautions were taken for operating radiographic equipment within inches of a large body of saltwater (6).
As of 14 February 2006, the ~5-month-old calf was in good body condition and continued to be nursed and cared for by the allomother. CBC and blood chemistry data were considered normal and IgG levels were higher than expected. This case will serve as a model for future intervention plans at our facility.
Click on the image to see a larger view.
| Figure 1. Parturition & calf electronic handheld database flow chart Navy Marine Mammal Program. | 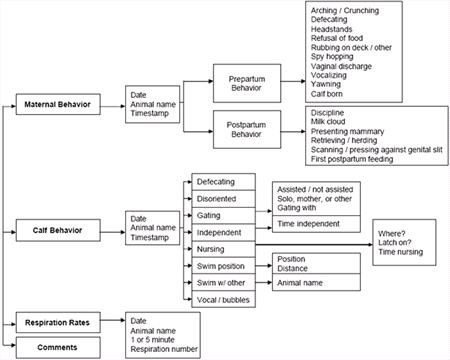
|
|
| |
Table 1. Comparison of routine CBC and serum chemistry mean values by age group: Tursiops truncatus, Jan 1999-Jan 2006.
|
Blood variable |
Mean value, neonates *(All) n=30 |
Mean value, neonates* (routine) n=13 |
Mean value, calves* (routine) n=166 |
Mean value, adults* (routine) n=2,138 |
p-value |
Significant differences among routine sampling groups (p-value < .05) |
|
WBC (/ul) |
8,373 |
6,431 |
9,002 |
8,368 |
0.0002 |
Calf > adult > neonate |
|
HCT (%) |
40.4 |
38.6 |
42.9 |
41.8 |
< .0001 |
Calf > adult > neonate |
|
Platelets (/ul) |
205.3 |
176.5 |
137.7 |
104.0 |
< .0001 |
Neonate > calf > adult |
|
Neutrophils (/ul) |
5,074 |
3,769.0 |
5,898.0 |
6,006.0 |
0.76 |
- |
|
Lymphocytes (/ul) |
2,524 |
2,182.0 |
1,779.0 |
1,181.0 |
< .0001 |
Calf, neonate > adult |
|
Monocytes (/ul) |
406 |
164 |
345 |
289.0 |
0.009 |
Calf > adult |
|
Eosinophils (/ul) |
429 |
327 |
978 |
1,132.0 |
< .0001 |
Adult > calf > neonate |
|
Glucose (mg/dl) |
142.3 |
137.0 |
107.9 |
114.3 |
< .0001 |
Neonate > adult > calf |
|
BUN (mg/dl) |
51.1 |
52.2 |
47.6 |
47.4 |
0.24 |
- |
|
Creatinine (mg/dl) |
0.8 |
0.9 |
1.2 |
1.5 |
< .0001 |
Adult > calf > neonate |
|
Uric acid (mg/dl) |
0.1 |
0.1 |
0.4 |
0.3 |
0.0007 |
Calf > adult, neonate |
|
Sodium (mEq/l) |
154.6 |
154.2 |
155.1 |
155.4 |
0.09 |
- |
|
Potassium (mEq/l) |
4.1 |
4.0 |
3.8 |
3.8 |
0.03 |
Neonate > adult |
|
Chloride (mEq/l) |
112.8 |
113.9 |
119.4 |
119.5 |
< .0001 |
Adult, calf > neonate |
|
CO2 (mEq/l) |
29.5 |
27.2 |
24.6 |
23.8 |
0.08 |
- |
|
Protein (g/dl) |
5.9 |
5.9 |
6.4 |
7.1 |
< .0001 |
Adult > calf > neonate |
|
Albumin (g/dl) |
4.2 |
4.2 |
4.1 |
4.3 |
< .0001 |
Adult > calf |
|
Globulins (g/dl) |
1.7 |
1.7 |
2.4 |
2.8 |
< .0001 |
Adult > calf > neonate |
|
Cholesterol (mg/dl) |
198.4 |
205.2 |
231.8 |
225.6 |
0.12 |
- |
|
Triglyceride (mg/dl) |
103.8 |
97.1 |
71.6 |
117.6 |
< .0001 |
Adult > calf |
|
Calcium (mg/dl) |
10.0 |
10.0 |
9.4 |
9.1 |
0.04 |
- |
|
Inorg Phos (mg/dl) |
6.3 |
6.1 |
5.4 |
4.9 |
< .0001 |
Calf, neonate > adult |
|
Alk Phos (u/l) |
2,499.0 |
2,630 |
705.0 |
314.2 |
< .0001 |
Neonate > calf > adult |
|
LDH (u/l) |
490.3 |
472.4 |
377.5 |
429.2 |
0.007 |
Adult > calf |
|
AST (u/l) |
108.4 |
107.7 |
227.1 |
263.7 |
< .0001 |
Adult > calf > neonate |
|
ALT (u/l) |
21.5 |
24.1 |
34.9 |
40.8 |
0.03 |
- |
|
GGT (u/l) |
29.3 |
26.2 |
29.9 |
72.8 |
0.001 |
Adult > calf |
|
Iron (ug/dl) |
287.5 |
260.2 |
190.0 |
230.5 |
0.0003 |
Adult > calf |
|
CPK (mu/ml) |
449.3 |
469.3 |
161.0 |
116.1 |
< .0001 |
Neonate > calf > adult |
|
ESR (60 min) |
10.9 |
8.6 |
9.5 |
12.3 |
0.04 |
- |
* Neonate = 0-1 Year Old and Survived > 1 year; Calf = > 1 to 5 Years Old; Adult = > 5 Years Old
Gray shading = Routine + non-routine samples; not used for group comparison analyses
Acknowledgments
The authors thank the training staff, veterinary technicians, researchers, and veterinarians who have worked with the NMMP breeding program over the years. In particular, we thank Dr. Mark Xitco, MAJ Brad Blankenship, and Dr. Sam Ridgway, as well as Mark Beeler, Joy Rothe, Elaine Allen, Mark Patefield, Brit Swenberg, Kathy Timon, and Beau Richter.
References
1. Ruiz C, Jacobson E, Nollens H, Wong S, Smith C, Jensen E. 2006. Development and implementation of a competitive ELISA for the quantification of IgG in neonate dolphins, Tursiops truncatus. IAAAM Proceedings.
2. Mann J, Smuts BB. 1998. Natal attraction: allomaternal care and mother-infant separations in wild bottlenose dolphins. Anim Behav 55 (5): pp.1097-1113.
3. Tavolga MC, Essapian FS. 1957. The behavior of the bottlenose dolphin Tursiops truncatus: mating, pregnancy, parturition and mother-infant behavior. Zoologica 421: pp. 11-31.
4. Leatherwood S. 1977. Some preliminary impressions on the numbers and social beha vior of free-swimming bottlenose dolphin calves. In: Breeding dolphins: present status, suggestions for the future. US Marine Mammal Commission. Eds. Ridgway SH and Benirschke K. Report No. MMC-76/07: pp. 143-158.
5. Wells RS. 1991. The role of long-term study in understanding the social structure of a bottlenose dolphin community. In: Dolphin societies: discoveries and puzzles. Eds. Norris KS, Wursig B, Wells RS, Wursig M. pp.199-225.
6. Jensen ED, Smith CR, Blankenship BA, Smith LE, Dold CM, and Ridgway SH. 2006. Digital radiography for pulmonary evaluation of bottlenose dolphins. IAAAM Proceedings.