Department of Large Animal Clinical Sciences, Veterinary College, University of Florida
Gainsville, FL, USA
Mammary Tumours
Contrary to the dogs where they are the most common tumours in the female, mammary tumours in cats are the third most common tumours after lymphomas and skin tumours. Their incidence is around 50% less than in dogs but they are still highly significant. Even if a breed predisposition is not obvious, breeds like Siamese are said to have a higher incidence (twice as high) of mammary neoplasia than other breeds. Incidence of mammary tumours in cats is estimated to be around 0.25/1000/year, they represent 14% of all tumours and 76% of the reproductive system tumours.
Age at detection is higher in queens than for all mammary tumours in dogs. However, it becomes similar to the age of detection in dogs if we consider only malignant tumours, with detection around 10 or 11 years of age (elderly animals). There is an obvious risk associated with age since there is an incidence of more than 2/1000 animals per year once the animal reaches 10 years old or more.
Endocrine influence and cyclical activity effects on mammary tissue are now well described and mammary tumours are more often detected in intact animals than in neutered ones. There are approximately seven times more mammary tumours in entire queens than in spayed females, but early ovariectomy does not completely suppress the risk like it does in female dogs. Indeed, detection of tumours in spayed females or in males is not exceptional in cats. Like in dogs, however, the earlier the spay the better. Indeed, in a study trying to determine the relation between age at spaying and incidence of mammary neoplasia, intact cats were significantly over-represented (odds ratio (OR) = 2.7, confidence interval (CI) = 1.4-5.3, P <0.01) in the mammary carcinoma population while cats spayed prior to 6 months of age had a 91% reduction in the risk of mammary carcinoma development compared with intact cats (OR = 0.09, CI = 0.03-0.24). Those spayed prior to 1 year old had an 86% reduction in risk (OR = 0.14, CI = 0.06-0.34). Like in dogs, regular administration of correlation with high doses of progestin has significant effects on the development of mammary lesions while pregnancy has been proposed to have a protective effect. Cycle irregularities, even if less often observed than in dogs, may also have a negative impact on the development of the mammary tumours. The induction role of pseudopregnancy on mammary tumours, which has been clearly demonstrated in dogs, remains unclear in cats.
Interestingly, the presence of oestrogen and progesterone receptors is detected in around 30% of cases, even mammary tumours accompanied by metastases. This is significantly more than in humans and totally different from the situation in female dogs where metastases are rarely positive for oestrogen or progesterone receptors.
Feline leukaemia virus (FeLV) particles have been detected in around 30% of mammary tumours. Their role has not yet been identified.
Clinical Signs of Mammary Tumours in the Queen
Generally the animals are presented to the veterinary surgeon for the presence of a mass noticed in the mammary chain. The masses are most often detected in the first anterior glands, and they are firm and nodular. Adhesions as well as signs of invasion of the surrounding tissues may often be present. Ulcerations may appear when the masses are of significant size or when the interval between detection and presentation is long. More than 50% of cats have several tumours at the time of presentation. These can be extensions of the primary one or totally different tumours evolving at the same time.
Mammary tumours in cats are generally malignant with the following frequencies:
 Carcinomas 86% (89% adenocarcinoma; 11% carcinoma)
Carcinomas 86% (89% adenocarcinoma; 11% carcinoma)
 Sarcomas 0.8%
Sarcomas 0.8%
 Benign 12%
Benign 12%
The high incidence of carcinomas explains why at the time of detection the lesions are generally multiple, large, poorly demarcated and invasive (50-90% develop metastases very quickly).
Less common tumours such as mucinous carcinoma or inflammatory carcinoma have also been described in the feline species. Mucinous carcinoma of the mammary gland is a rare tumour characterised by excessive mucin production. Of 656 cases of feline mammary neoplasms and dysplasias, 3.2% were found to be mucin-producing tumours in a recent study. Inflammatory carcinoma is a special type of locally advanced mammary cancer that is associated with particularly aggressive behaviour and poor prognosis. The dog was considered the only natural model for this pathology. However, spontaneous inflammatory mammary carcinoma was recently described in three cats.
Pathogenesis of Mammary Tumours
As stated in the introduction, numerous factors are involved in the development of mammary tumours in cats as in dogs, among which oestrogen and progesterone from the cyclical ovarian activity, exogenous progestin administration for contraception, growth hormone (GH) (induced locally when mammary tissue is under the influence of progesterone or progestins) as well as growth factors play a role (Figure 1).
The role of prolactin (PRL) in pathogenesis of mammary tumours in cats is still unclear.
Progesterone receptors and GH have been detected homogeneously throughout the lesions in cat mammary tumours while insulin-like growth factor I (IGF-I) was detected in 77% of the cases. Recent results suggest that ligand-activated progesterone receptors may induce the local synthesis of GH, which in turn may exert its proliferative action directly and also indirectly through the production of other growth factors, such as IGF-I, in an autocrine/paracrine manner.
Through promoting tumour proliferation and an 'angiogenic shift', growth factors such as vascular endothelial growth factor (VEGF) and the VEGF receptor/KDR may play a role in malignant transformation and tumour progression.
Prognosis
The prognosis can be established from a clinical point of view by using the TMN system (tumour, metastasis, nodes) with adjusting factors like history of rapid growth, development during the oestrous cycle, invasion, presence of metastases or ulcerations, all those being of poor prognosis.
Click on the image to see a larger view.
| Figure 1 | 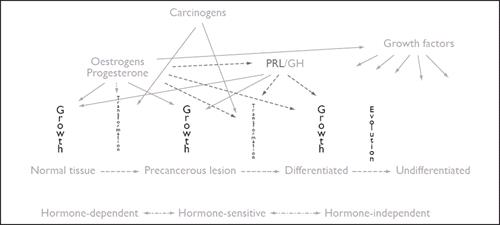
Pathogenesis of mammary tumours in cats. GH, growth hormone; PRL, prolactin. |
|
| |
The TMN system:
 T = primary tumour
T = primary tumour
 T0: no tumour (cyst)
T0: no tumour (cyst)
 T1: <3 cm, mean survival time (MST) over 2-3 years
T1: <3 cm, mean survival time (MST) over 2-3 years
 T2: between 3 and 5 cm, MST <2 years
T2: between 3 and 5 cm, MST <2 years
 T3: >5cm, MST <6 months
T3: >5cm, MST <6 months
 NB: mean interval between detection and death if no treatment is around 1 year!
NB: mean interval between detection and death if no treatment is around 1 year!
 M = distant metastasis (lung radiography is absolutely needed!)
M = distant metastasis (lung radiography is absolutely needed!)
 M0
M0
 M1 (MST <6 months).
M1 (MST <6 months).
 N = lymph nodes
N = lymph nodes
 N0
N0
 N1 ipsilateral lymph node, MST <6 months
N1 ipsilateral lymph node, MST <6 months
 N2 bilateral, MST even shorter
N2 bilateral, MST even shorter
Tumour size alone is of limited prognostic value in cats.
A full pre-surgical work-up including complete blood count (CBC), chemistry, urinalysis and thoracic radiographs should be performed prior to any therapeutic decisions. Regional lymph nodes should be evaluated by palpation and fine needle aspiration for presence of metastasis. Biopsies of the tumours are generally not recommended because of the high probability of malignancy and risk of dissemination. Post-surgery tissue histopathology is always the best kind of study one can make and would allow a precise diagnosis of the different types of lesions and an accurate prognosis to be made.
As is the case in humans, there is now support for a relationship between the expression of Stat3 (signal transducer and activator of transcription 3) and malignancy. Stat3 expression may become a standard test for prognosis after surgical removal of the lesions in cats and dogs.
Similarly, the presence of oestrogen receptors or progesterone receptors at the cellular level has been used to improve the prognosis and to help with the therapeutic decision. When tissues are positive for oestrogen receptors in humans, anti-oestrogen treatments may be established. The expression of oestrogen receptors was significantly higher in healthy tissues and in adenoma than in malignant lesions, and the levels of progesterone receptors was increased in fibroadenomatous changes and in 'in situ' carcinomas but was decreased in invasive carcinomas. Oestrogen receptor-negative tumours had the poorer prognosis, but were less frequent than in other species. The high percentage of progesterone receptor-positive feline carcinomas suggests a possible role of progesterone in promoting early tumour cell growth in queens. These observations may open new doors to therapeutics in cats as in other species.
Treatment of MTS in the Queen
Aggressive surgery is advised in all cases, even if the prognosis is always hindered by the highly malignant nature of the lesions. Total removal of the affected or both mammary chains is recommended, reducing the probability for extension and metastases. The mammary tissue as well as the lymph nodes should be removed. The author is always tempted to remove both chains at the same time in order to:
 Prevent risks of extension during the interval between the two surgeries
Prevent risks of extension during the interval between the two surgeries
 Reduce the immune depressive effects of the surgeries which will promote tumour development and may prevent a second surgery
Reduce the immune depressive effects of the surgeries which will promote tumour development and may prevent a second surgery
 Prevent the owner from changing his decision in between the two surgeries
Prevent the owner from changing his decision in between the two surgeries
The aggressive surgery significantly reduces the risk of recurrence of the lesions and increases the overall survival time. The lymph nodes should always be submitted to pathology after the surgery with the lesions. Indeed around 50% of the lymph nodes end up with a positive diagnosis even if the fine needle biopsy was negative.
Some adjuvant therapies may be instituted with some good success rates and increased survival time:
 Chemotherapy in association with surgery or alone if surgery is not possible. Doxorubicin alone or in combination with cyclophosphamide is well described: Doxorubicin at 25-30 mg/m2 i.v. + cyclophosphamide at 50-100mg/m2 days 3, 4, 5, 6 every 3 weeks. Some recent studies confirm the beneficial effects of this treatment.
Chemotherapy in association with surgery or alone if surgery is not possible. Doxorubicin alone or in combination with cyclophosphamide is well described: Doxorubicin at 25-30 mg/m2 i.v. + cyclophosphamide at 50-100mg/m2 days 3, 4, 5, 6 every 3 weeks. Some recent studies confirm the beneficial effects of this treatment.
 The use of COX-2 inhibitors has been advocated, but is still controversial. Cyclooxygenase (COX) enzymes catalyse the synthesis of prostaglandins and exist as two isoforms, COX-1 and COX-2. COX-2 is a potent inducible mediator of inflammation. COX-2 is also upregulated in several human tumours and in canine squamous cell, renal cell and transitional cell carcinomas, prostate adenocarcinomas and intestinal neoplasia. In a recent study, no COX-immunoreactivity was found in mammary carcinomas, suggesting low potential for COX-2 inhibitors as anticancerous agents for those tumours. However, in another study, COX-2 was significantly expressed in mammary tissues during tumourigenesis and its expression was associated with a poorer prognosis in bitches and queens. The correlation between COX-2 expression and angiogenesis provided support for a potential role of COX-2 inhibitors for the prevention and the treatment of feline mammary carcinomas via their anti-angiogenic properties. In conclusion, further studies are still needed to clarify the role of COX-2 inhibitors in cats.
The use of COX-2 inhibitors has been advocated, but is still controversial. Cyclooxygenase (COX) enzymes catalyse the synthesis of prostaglandins and exist as two isoforms, COX-1 and COX-2. COX-2 is a potent inducible mediator of inflammation. COX-2 is also upregulated in several human tumours and in canine squamous cell, renal cell and transitional cell carcinomas, prostate adenocarcinomas and intestinal neoplasia. In a recent study, no COX-immunoreactivity was found in mammary carcinomas, suggesting low potential for COX-2 inhibitors as anticancerous agents for those tumours. However, in another study, COX-2 was significantly expressed in mammary tissues during tumourigenesis and its expression was associated with a poorer prognosis in bitches and queens. The correlation between COX-2 expression and angiogenesis provided support for a potential role of COX-2 inhibitors for the prevention and the treatment of feline mammary carcinomas via their anti-angiogenic properties. In conclusion, further studies are still needed to clarify the role of COX-2 inhibitors in cats.
Future approaches may involve:
 Investigation of the interest of antiprolactinic agents in cats as they were proven to be helpful in dogs and humans.
Investigation of the interest of antiprolactinic agents in cats as they were proven to be helpful in dogs and humans.
 Vitamin B6 treatment, locally or parenterally. Indeed, Vitamin B6 suppresses growth of one feline mammary tumour cell line in vitro in a dose-dependent manner.
Vitamin B6 treatment, locally or parenterally. Indeed, Vitamin B6 suppresses growth of one feline mammary tumour cell line in vitro in a dose-dependent manner.
 It has recently been suggested that the chemokine receptor CXCR4 and its ligand SDF-1 (CXCL12) promote metastasis of various cancers in cats as in humans. fCXCR4 may be a future therapeutic target for feline mammary tumours.
It has recently been suggested that the chemokine receptor CXCR4 and its ligand SDF-1 (CXCL12) promote metastasis of various cancers in cats as in humans. fCXCR4 may be a future therapeutic target for feline mammary tumours.
 Combination of chemotherapy and electrotherapy. Recent studies have shown that electrochemotherapy with cisplatin is an effective, safe and simple treatment of different histological types of mammary tumours in cats and dogs.
Combination of chemotherapy and electrotherapy. Recent studies have shown that electrochemotherapy with cisplatin is an effective, safe and simple treatment of different histological types of mammary tumours in cats and dogs.
Fibroadenomatosis
This is also called mammary hypertrophy, mammary dysplasia, fibroepithelial hyperplasia and fibro-glandular mammary hypertrophy. In fact, fibroadenomatosis is not a cancer but a pseudotumoural disease, most often observed in the young queen. It can also be observed in older animals but extension and development will always be more marked in young animals than in elderly patients (in contrast to mammary tumours). Lesions are more generalised in young queens while they are more localised in older ones.
The pathology is characterised by often symmetrical, bilateral and non-painful mammary masses in young animals. The lesions start from the posterior mammary glands and extend slowly and progressively to the cranial ones (again in contrast to mammary tumours). In older queens (>5 years) the masses are more often localised to one gland and may be similar to mammary tumours. No milk secretion is noticed in the affected glands, the lesions are firm, most often homogeneous, but can sometimes be nodular; the changes may have a rapid evolution, but are reversible. The glands are not painful, at least as long as the nodules are not ulcerated by contact with the hard structures of the environment. The lesions seem to be due to a hypersensitivity to progesterone. Fibroadenomatosis can thus be observed:
 After the first exogenous progestin treatments (oral or parenteral). This implies a male can develop the disease if he is receiving progestins for any reason (e.g., delmadinone acetate to reduce libido for example).
After the first exogenous progestin treatments (oral or parenteral). This implies a male can develop the disease if he is receiving progestins for any reason (e.g., delmadinone acetate to reduce libido for example).
 After the first ovulation in a mated (pregnant) or not mated (pseudopregnant) queen and development of a functional corpus luteum.
After the first ovulation in a mated (pregnant) or not mated (pseudopregnant) queen and development of a functional corpus luteum.
Treatment
 Ovariectomy if the condition has developed because of an endogenous corpus luteum (pregnancy or pseudopregnancy) and the cat is not intended to breed. The lesions generally regress in a few days or weeks.
Ovariectomy if the condition has developed because of an endogenous corpus luteum (pregnancy or pseudopregnancy) and the cat is not intended to breed. The lesions generally regress in a few days or weeks.
 If the animal is a breeding queen:
If the animal is a breeding queen:
 Induce luteolysis using anti-prolactinic agent such as cabergoline and/or prostaglandins (PGF2alpha). This is indicated only if the fibroadenomatosis occurred after an ovulation. So, to confirm the presence of endogenous corpus luteum, a progesterone testing must be performed.
Induce luteolysis using anti-prolactinic agent such as cabergoline and/or prostaglandins (PGF2alpha). This is indicated only if the fibroadenomatosis occurred after an ovulation. So, to confirm the presence of endogenous corpus luteum, a progesterone testing must be performed.
 Treat with progesterone antagonist (aglepristone, e.g., Alizine) if the condition was induced by progestin administration. If oral contraceptives were used, stop their administration and treat for at least 3 weeks. A long duration of treatment (up to 4 months) may be required if fibroadenomatosis was induced by a depot injectable formulation.
Treat with progesterone antagonist (aglepristone, e.g., Alizine) if the condition was induced by progestin administration. If oral contraceptives were used, stop their administration and treat for at least 3 weeks. A long duration of treatment (up to 4 months) may be required if fibroadenomatosis was induced by a depot injectable formulation.
In any case, the use of aglepristone is recommended to hasten the resolution of the lesions and can thus be combined with ovariectomy or with PGF2alpha. If the breeder would like to keep the ongoing pregnancy, it may be possible to allow the pregnancy to go on, but only if lesions are not too severe and not ulcerated. Indeed, they will regress spontaneously after queening and a normal lactation process can occur in the non-affected mammary glands. However, those affected queens are not good candidates for breeding, as the condition will likely recur at each pregnancy.
A surgical treatment involving affected mammary gland removal is not generally required; the only exceptions will be severely ulcerated lesions or no regression after aglepristone treatment.
Acknowledgement
BSAVA thanks John Verstegen DVM MSc PhD DipECAR for his assistance with these notes.