Therapy for coagulation defects is required when there is clinical evidence of bleeding (e.g., epistaxis, gastrointestinal bleeding) or when bleeding might be expected (e.g., following anticoagulant rodenticide toxicity or prior to surgery in a dog with a known coagulopathy). This lecture aims to provide a basic understanding of haemostasis and the common causes of coagulation defects, before discussing how, when and why we treat them.
Normal Haemostasis
Haemostasis can be divided into three separate phases which happen sequentially whenever vascular damage occurs. The first phase (primary haemostasis) leads to the rapid formation of a platelet plug over the area of damage. Platelets cannot adhere to the damaged area directly and need von Willebrand's factor to adhere to the damaged area. Once the platelet plug has formed it is stabilised by a mesh of fibrin formed by the coagulation cascade (secondary haemostasis--Figure 1). Classically secondary haemostasis has been divided into the intrinsic pathway (triggered when damage exposes negatively charged surfaces such as collagen) and the extrinsic pathway (triggered when damage leads to tissue factor production); however, more recently it has become apparent that there is more interplay between the coagulation factors than first thought and a more integrated system of initiation and propagation has been proposed. Once the damaged vessel has been repaired the clot is removed by fibrinolysis (tertiary haemostasis).
| Figure 1 | 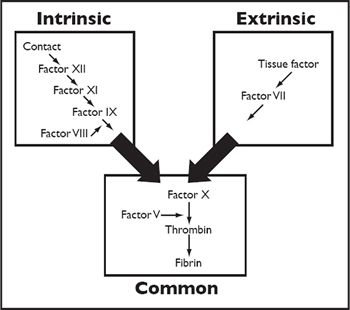
The coagulation cascade comprises the intrinsic and extrinsic pathways, which both feed into the common pathway. |
|
| |
Coagulation Defects
Coagulation defects can be divided into two groups depending on whether the coagulation defect lies with the production of a platelet plug (primary haemostatic defects) or with its stabilisation by a fibrin mesh (secondary haemostatic defects). This difference is important as the treatment for each type of defect is very different. Clinical examination can often provide clues as to whether primary or secondary defects are present, although sometimes problems overlap and laboratory investigations are needed. Primary haemostatic defects result in bleeding from mucosal surfaces and can result in cutaneous haemorrhage (petechial and ecchymotic haemorrhages), gastrointestinal bleeding (such as melaena), nasal bleeding (epistaxis) or urinary tract bleeding (haematuria). Secondary haemostatic defects result in haemorrhage from large blood vessels and cause large ecchymoses, haematomas and bleeding into body cavities.
Haemostatic Tests
Primary haemostasis relies on normal platelet numbers, normal platelet function and adequate levels of von Willebrand's factor. Platelet numbers can be checked by routine haematology and confirmed manually by examining a fresh blood smear. The blood smear is checked for platelet clumps and the platelets per field counted in the monolayer behind the feathered edge. Here one platelet per x100 field is equivalent to approximately 15 x 109/l platelets. Platelet function and von Willebrand's factor levels can be assessed by the buccal mucosal bleeding time (BMBT). Platelet numbers should be checked first as thrombocytopenia will lead to marked bleeding. The BMBT is performed by making an incision on the oral mucosa with a standard device such as the Simplate II®. The upper lip is usually folded and tied back to allow the incision to be made, once made excessive bleeding is absorbed using a swab, taking care not to touch the actual incision site. Normal BMBT times are approximately 1-2½ minutes for the cat and 1½-4½ minutes in the dog. Results are prolonged with inadequate platelet numbers, reduced platelet function (thrombocytopathia), reduced von Willebrand's factor and vascular defects such as vasculitis.
Secondary haemostasis relies on adequate levels of coagulation factors to allow stabilisation of the platelet plug by a fibrin mesh. This can crudely be measured using the whole blood coagulation time (WBCT), which assesses the extrinsic and common pathways. Blood is taken into a warm glass tube and tilted every 30 seconds until it clots. At 37°C this should normally occur within 6-7 minutes. An activated clotting time (ACT) is a more sensitive way to examine the extrinsic and common pathways and uses a commercial tube with a clay activator. Blood is taken into the tube and whilst being warmed at 37°C the tube is tilted every 10 seconds until a clot is seen. Blood should clot within 50-75 seconds in cats and 60-120 seconds in dogs. More detailed coagulation times can be run at external laboratories or on bedside analysers such as the SCA2000®. The prothrombin time (PT) allows investigation of the extrinsic pathways of coagulation, with the activated partial thromboplastin time (aPTT) allowing investigation of the intrinsic pathway. These tests are run on citrated blood samples and test results >25% greater than the control samples are abnormal.
Primary Haemostatic Defects
Defects in any one of the three key components of primary haemostasis will lead to a failure in platelet plug formation. Primary haemostatic defects therefore fall into three categories: decreased platelet numbers (thrombocytopenia), abnormal platelet function (thrombocytopathia) and von Willebrand's factor deficiency.
 Thrombocytopenia. This is the commonest cause of coagulation defects in small animal practice and is confirmed by documenting a low platelet count. Spontaneous bleeding does not usually occur until the platelet count drops below 20 x 109/l; however, this varies between different animals. Thrombocytopenia can be caused by decreased platelet production, increased platelet destruction or increased platelet consumption.
Thrombocytopenia. This is the commonest cause of coagulation defects in small animal practice and is confirmed by documenting a low platelet count. Spontaneous bleeding does not usually occur until the platelet count drops below 20 x 109/l; however, this varies between different animals. Thrombocytopenia can be caused by decreased platelet production, increased platelet destruction or increased platelet consumption.
 Thrombocytopathia. Platelet dysfunction is usually associated with non-steroidal antiinflammatory drugs (NSAIDs) such as aspirin, but can also be seen with renal and hepatic failure and neoplasia. Thrombocytopathia is confirmed by normal platelet numbers but an increased BMBT. Spontaneous bleeding is not normally seen.
Thrombocytopathia. Platelet dysfunction is usually associated with non-steroidal antiinflammatory drugs (NSAIDs) such as aspirin, but can also be seen with renal and hepatic failure and neoplasia. Thrombocytopathia is confirmed by normal platelet numbers but an increased BMBT. Spontaneous bleeding is not normally seen.
 Von Willebrand's disease. Von Willebrand's factor (VWF) is needed to allow platelets to adhere to areas of vascular damage. Von Willebrand's disease is a relatively common hereditary disease in which low levels of VWF are present. These dogs usually present with signs of a primary coagulopathy at a young age and diagnosis is confirmed by measuring VWF levels.
Von Willebrand's disease. Von Willebrand's factor (VWF) is needed to allow platelets to adhere to areas of vascular damage. Von Willebrand's disease is a relatively common hereditary disease in which low levels of VWF are present. These dogs usually present with signs of a primary coagulopathy at a young age and diagnosis is confirmed by measuring VWF levels.
Secondary Haemostatic Defects
Deficiencies of clotting factors lead to secondary coagulation defects. These can be hereditary defects resulting in the deficiencies of single clotting factors such as haemophilia A (factor VIII deficiency) or more generalised diseases caused by decreased production (e.g., liver failure or anticoagulant rodenticide toxicity) or consumption of the clotting factors (e.g., disseminated intravascular coagulation (DIC)).
 Haemophilia A. This is a rare sex-linked hereditary condition seen in young male animals. It will lead to elevation in aPTT and is confirmed by analysis of factor VIII.
Haemophilia A. This is a rare sex-linked hereditary condition seen in young male animals. It will lead to elevation in aPTT and is confirmed by analysis of factor VIII.
 Anticoagulant rodenticide toxicity. Rat poisons, such as warfarin and brodifacoum, reduce the body's concentration of active vitamin K. Vitamin K is essential in the production of clotting factors II, VII, IX and X, hence toxicity can result in haemorrhage. In early poisoning the PT may be elevated due to the shortest half-life of factor VII; however, by the time most animals present both aPPT and PT are usually markedly elevated.
Anticoagulant rodenticide toxicity. Rat poisons, such as warfarin and brodifacoum, reduce the body's concentration of active vitamin K. Vitamin K is essential in the production of clotting factors II, VII, IX and X, hence toxicity can result in haemorrhage. In early poisoning the PT may be elevated due to the shortest half-life of factor VII; however, by the time most animals present both aPPT and PT are usually markedly elevated.
 Liver failure. The liver is responsible for the production of most coagulation factors, thus liver failure can result in increased coagulation times. Although liver failure rarely results in spontaneous haemorrhage, it is certainly a consideration prior to surgical or biopsy techniques.
Liver failure. The liver is responsible for the production of most coagulation factors, thus liver failure can result in increased coagulation times. Although liver failure rarely results in spontaneous haemorrhage, it is certainly a consideration prior to surgical or biopsy techniques.
Disseminated Intravascular Coagulation
DIC occurs as a result of generalised activation of the coagulation system; thus it is both a primary and a secondary haemostatic disease. Laboratory tests will usually reveal thrombocytopenia and elevated coagulation times. Many diseases can trigger DIC and these include heat stroke, neoplasia (especially haemangiosarcoma) and infection (especially sepsis).
Treatment of Coagulation Defects
Treatment of coagulation defects focuses on treating the underlying condition, correcting depleted clotting factors/platelets and providing appropriate supportive care. In many cases treating the underlying cause is not possible (e.g., von Willebrand's disease); however, in some circumstances specific treatments are indicated such as immunosuppressant therapy in immune-mediated thrombocytopenia and vitamin K in anticoagulant rodenticide toxicity. Replacing depleted clotting factors and platelets relies on transfusion medicine; with component therapy becoming increasingly available to UK practices, products are being produced to allow replacement of specific haemostatic components.
 Whole blood. Whole blood transfusions provide clotting factors and platelets, as well as red blood cells. Thus whole blood is best given when both red cells and coagulation factors are needed. Whole blood contains platelets for 2-4 hours after collection, with factors V and VII being lost with 24 hours of storage. The number of platelets in whole blood is low, but whole blood transfusions may provide enough platelets to stop bleeding or allow surgery, in cases of thrombocytopenia.
Whole blood. Whole blood transfusions provide clotting factors and platelets, as well as red blood cells. Thus whole blood is best given when both red cells and coagulation factors are needed. Whole blood contains platelets for 2-4 hours after collection, with factors V and VII being lost with 24 hours of storage. The number of platelets in whole blood is low, but whole blood transfusions may provide enough platelets to stop bleeding or allow surgery, in cases of thrombocytopenia.
 Fresh frozen plasma (FFP). This is obtained from whole blood that has been spun, separated from red blood cells and frozen within 6 hours of collection. FFP contains all the clotting factors, but does not contain platelets. Transfusing FFP allows greater quantities of clotting factors to be given per volume when compared to blood, reducing the risks associated with volume and red cell overload.
Fresh frozen plasma (FFP). This is obtained from whole blood that has been spun, separated from red blood cells and frozen within 6 hours of collection. FFP contains all the clotting factors, but does not contain platelets. Transfusing FFP allows greater quantities of clotting factors to be given per volume when compared to blood, reducing the risks associated with volume and red cell overload.
 Cryoprecipitate. Cryoprecipitate is prepared by partially thawing and spinning FFP, allowing concentration of VWF and factor VIII in a small volume of fluid. This is partially useful to give dogs with von Willebrand's disease or haemophilia A prior to surgery.
Cryoprecipitate. Cryoprecipitate is prepared by partially thawing and spinning FFP, allowing concentration of VWF and factor VIII in a small volume of fluid. This is partially useful to give dogs with von Willebrand's disease or haemophilia A prior to surgery.