A.M. Oberbauer, PhD
Objectives of the Presentation
To provide an appreciation for the complexity surrounding investigations of the genetic mechanisms underlying canine epilepsy.
Overview of the Issue
Epilepsy is a disorder of the brain characterized by recurrent seizures. Seizures are caused by excessive and hypersynchronous neuronal discharge. That is, a seizure represents a disruption in normal nerve activity. In normal brain activity, neuronal information is transmitted by electrical impulses in a coordinated fashion. In a seizure, the electrical energy may have been released at inappropriate times and/or at a greater intensity than is necessary resulting in transient interference of normal nerve transmission. Depending upon the extent of the brain involved, seizures can be either partial (or focal) or generalized. This in turn, impacts the physical expression of the seizure that is observed producing psychic or motor alterations. Causes of epilepsy include trauma, toxic insult, infection, or structural abnormalities. If no underlying cause for epilepsy is identified, the condition is considered idiopathic.
Epilepsy affects all human populations at a rate of approximately 1%. Many other species also experience epilepsy including cats, horses, goats, cattle, non human primates, and rodents (Chandler, 2006). Epilepsy is especially common among dogs with an overall prevalence of 5% (Lohi et al., 2005). Some purebreds have a greater prevalence of repeated seizure activity, but all dog breeds, including non-purebred dogs, can have individuals with epilepsy. Given the general prevalence of epilepsy among domesticated canines and the relative higher prevalence within certain breeds, genetic contribution to the expression of epilepsy is expected. As an aside, epilepsy may be a consequence of domestication as wolves rarely exhibit seizures except under pharmacological treatment [personal communication, Dr. L. D. Mech, Senior Scientist with the Biological Resources Division, U.S. Geological Survey and an Adjunct Professor in the Department of Fisheries, Wildlife and Conservation Biology, and Ecology, Evolution and Behavior at the University of Minnesota].
Treatment of epilepsy is costly and often ineffective. Breeders are highly motivated to reduce the likelihood of producing puppies that will later be diagnosed with seizure episodes and have initiated studies to explore the genetic basis to the disorder. In addition, the epileptic seizures experienced by dog may offer insights into progression and potential therapeutics for the human condition. Sutter and Ostrander (2004) and Ellegren (2005) both emphasize the potential utility of dog breeds as models to dissect the genetics underlying complex human disorders. Thus, numerous investigators have been studying canine epilepsy at all levels: physiological, neurological, cellular, and molecular. Although the simplified genetic structure of certain dog breeds may be useful for understanding human disease, dog breeders want to reduce the prevalence of epilepsy in the dog. One of the first steps necessary to reduce the prevalence of epilepsy in a breed of dog is to characterize the genetic contribution and predict the mode of inheritance of the condition.
Many investigators have evaluated breeds of dogs known for having a higher than expected prevalence of epilepsy. Based on assessment of pedigrees and phenotypic data, the published predicted canine models of epilepsy inheritance have been consistent with multilocus modes that include autosomal recessive with incomplete penetrance (Srenk et al., 1994; Famula et al., 1997; Jaggy et al., 1998; Patterson et al., 2005; Casal et al., 2006), although a single locus model has been proposed for idiopathic epilepsy in Keeshonds (Hall and Wallace, 1996) and Vizslas (Patterson et al., 2003).
Our laboratory has been investigating the inheritance of repeated seizures that appear to have no underlying cause, and represent generalized seizures (myoclonic, clonic, tonic clonic). An acknowledged limitation of our research population is that the condition is owner reported; usually by an owner whose dog experienced a seizure and, following a veterinary examination, learns about idiopathic epilepsy or by a breeder long familiar with the condition of idiopathic epilepsy. The subtle nuances of a seizure are often not detected nor reported and the diagnosis can never be definitive. This is a difficulty associated with seizure classification and therefore, the study of canine epilepsy (Chander, 2006). Nevertheless, owners of particular breeds are well aware of the condition and their reports are remarkably similar. For our study we restrict classifying dogs as epileptic to those that the owner describes as exhibiting generalized seizures. Licht et al. (2002) and Podell (2004) have recommended the application of the human International League Against Epilepsy (ILAE) seizure classification system to dogs that had experienced seizures. A more accurate description of canine seizures could refine epilepsy research and yield better treatment for dogs; a consistent classification of seizures would improve the accuracy of heritability estimates and mode of inheritance predictions.
We have been accumulating generalized epileptic seizure data on Poodles (2116 dogs, 83 with repeated seizing activity), Giant Schnauzers (291 dogs, 25 with repeated seizing activity), English Mastiffs (630 dogs, 36 with repeated seizing activity), Belgian Sheepdogs (858 dogs, 117 with repeated seizing activity) and Belgian Tervuren (1064 dogs, 136 with repeated seizing activity). The prevalence of epilepsy among these admittedly biased samples ranges from ~4% to 13.6%. From the submissions we have organized dogs into familial structures to evaluate heritability. Figure 1 provides illustrations of portions of the pedigrees of submitted dogs. The expression of the seizing phenotype in the English Mastiff has an early profile of expression relative to other breeds. The average age of onset of the seizures in the English Mastiff is 29.2 months of age with 47% having their first seizure before 2 years of age and the oldest age for an English Mastiff to have begun seizuring is 57 months. That is in contrast to the Belgian Tervuren and Sheepdogs (where we have an abundance of data) where only 21% of the dogs have their first seizure prior to 2 years of age, Standard Poodles with a mean age of seizure onset of 42.7 months and 37.7% with their first seizure by 2 years of age, and Giant Schnauzers with an average age of onset of 36.2 months with 44% having their first seizure by 2 years of age. The very limited data for Toy and Miniature Poodles do not allow meaningful comparisons.
The heritability of seizures was estimated using a mixed model Bayesian analysis strategy in an ordered categorical threshold model. Narrow sense heritability was estimated with the SOLAR computational package using binary trait analysis. For heritability analyses, we have excluded young animals to preclude biasing the predictions. We wanted to avoid including dogs that may eventually seize but have not due to their young age. Therefore we "censored" the data to exclude dogs under the age of four years unless those dogs were categorized as repeatedly seizing. We statistically assessed the heritability of epilepsy in the Standard Poodle (the Poodle variety with substantial submissions) and the estimate of heritability for seizures was 0.59 with a standard error of 0.28. The heritability was not significantly different for males vs. females. We have also estimated heritability of multiple seizures in the Giant Schnauzer even though the total number of submitted dogs is low; the number of dogs that have seized repeatedly was fairly high and the dogs with seizing activity were highly related which enabled the evaluation. For example, for one family we had six generations and 23 of the affected dogs. In the data set we evaluated 285 Giant Schnauzers with known health status, 25 of which seized repeatedly. The estimate of heritability was 0.78 and was highly significant (p < 0.007). Sex was not a significant contributor to the seizing phenotype. Using data on 588 English Mastiffs with known health status and parents with 33 of those recorded as seizing repeatedly, the estimate of heritability was 0.69 with a standard error of 0.44. The high standard error equates to a non significant estimate of heritability (the significance level is p = 0.065 and while nearly reaching the p = 0.05 for classical significance it is considered only tending toward significance). Of interest, though possibly reflecting low numbers of dogs in our analyses, males were more likely to be afflicted with repeated seizures than females (p < 0.05). We have published the data on the Tervuren and Sheepdogs and the estimate of heritability of epilepsy ranged from 0.77-0.83. Our work in Belgians suggest the predicted mode of inheritance is polygenic but with a single gene of large effect influencing expression of the epileptic phenotype.
In most instances the putative mode of inheritance of epilepsy in breeds that have been studied is "complex" or "polygenic". Yet most analyses also suggest an autosomal recessive major locus with the complexity being additional loci regulating penetrance. As noted above, Ellegren (2005) considers the dog an extremely advantageous model for studying genetic disorders with a human counterpart: "each pure breed is an inbred, isolated genetic population with simplified genetic structures than can be linked to their physical traits." Sutter and Ostrander (2004) consider dog breeds to be ideal for unraveling disease alleles of complex disorders and cite the findings of quantitative loci associated with skeletal morphometrics in the Portuguese Water Dog.
While a dog model as whole is valuable for the identification of genes causing a disorder, caution must also be applied to prevent global generalization. Breeds are clearly distinct from one another although certain breeds do cluster together (Sutter & Ostrander, 2004). The importance of that fact lies in the basis for the distinctness: nucleotide heterozygosity and single nucleotide polymorphisms (SNP). Given the variability of the underlying DNA between breeds it is necessary to consider that dissimilar breeds may have different and distinct mutations that affect the expression of epilepsy. This is especially true given the nature of seizures and their inexact classification by dog owners and general practice veterinarians. This complicates the search for "the gene for epilepsy" that all breeders and dog owners desire.
However, Lohi et al (2005) identified a specific mutation associated with a rare form of epilepsy seen in miniature wirehaired dachshunds (MWHD). This research group noticed congruence between the seizure characteristics in the MWHD and a severe seizure disorder in humans, Lafora disease whose genetic mutation had been mapped. Taking advantage of the recent canine genome sequencing project and its synteny with the human genome, the investigators identified a mutation in the Epm2b gene (also known as NHRLC1) of affected MWHDs. This represented the identification of the first gene involved in any canine epilepsy, albeit a very restricted form. Perhaps most telling from this group's research is the number of different mutations and genetic alterations identified within two genes, each of which results in the same epileptic phenotype of Lafora disease. This corroborates the hypothesis put forth by Licht et al. (2002) that different parental lines within a breed may manifest epilepsy through distinct genetic mechanisms. This last underscores the challenge faced by investigators searching for the genetic basis for canine epilepsy.
Nevertheless, the rapid advances in the canine genome with an increase in tools available for genetic searches should make it possible to identify alterations in the genome that underlie epilepsy. The now classical approach is to do a genome wide association study using microsatellite markers evenly spaced across the genome. In the Belgian Tervuren and Sheepdog, we have done a genome wide association study using microsatellite markers. In the population as a whole, particular alleles were more prevalent in dogs with seizures than in dogs that did not based on a linkage disequilibrium analysis. We then constructed extensive pedigrees and did haplotype analyses on the related dogs in which seizures segregate. In evaluating the haplotypes, there was no clear association between the haplotypes and the seizures across families although within one very small family, the haplotype transmission appeared strong. The absence of haplotype linkage may reflect phenocopy; overall certain families exhibit seizure characteristics similar to other families and the genetics similarly reflect the influence of a single autosomal recessive gene, the actual gene that is altered may differ between families as has been suggested by Licht et al., (2002). That is, while as a whole, the population of Tervuren and Sheepdogs exhibit a major gene inherited as an autosomal recessive, the precise mutation or gene may differ in different families even though generating a remarkably similar phenotype. We have now focused our attention on small, single families rather than on the broader more extensive family; that is we are looking at the subset families within the larger family we assembled.
Based on the indication of a significant genetic contribution to the expression of seizures as indicated by the high heritability estimates in the other breeds, we initiated a search for genetic linkage between the seizing phenotype and a particular chromosomal region. We approached this by doing a homozygosity analysis in which 8 highly unrelated dogs are screened using the minimal screening set II of 327 microsatellite markers (MSS-2, offering 9 Mb coverage) markers. This approach presumes an autosomal recessive mode of inheritance and that the unrelated individuals only share the DNA that encodes (or is linked to) the region causing the mutation responsible for the seizuring phenotype. We selected 8 unrelated English Mastiffs and 8 unrelated Standard Poodles. For the English Mastiffs, the chromosomes showing some association were 1, 2, 5, 7, 8, 9, 10, 11, 18, 23, 26, 27, 30, 31, 32, 33, and 34. The high number of chromosomes exhibiting homozygosity may reflect that the breed is more homozygous as a whole and that the homozygosity approach will not offer any improvement over a generalized genome scan to identify linkage. In the Standard Poodles, the chromosomes showing some association were 1, 3, 5, 12, 23, and 32. It is interesting however that both the Standard Poodle and the English Mastiff demonstrated homozygosity on chromosomes 1, 5, 23, and 32. This approach utilizes the linkage disequilibrium that exists within dog breeds. The genetic material surrounding a mutation should be ancestral and held in common among dogs that carry the altered DNA. However, certain dog breeds may have experienced severe bottlenecks and the linkage disequilibrium may be extensive rendering the classical approach less effective meaning more and more variable markers must be used. The newly available SNP chip offers the best approach to mapping complex disease traits.
In summary, the complex nature of canine epilepsy will prove challenging with respect to determining the underlying genetic mechanisms responsible. However, the advancements in the DNA technologies will enable scientists to unravel regions of the chromosome regulating this debilitating disorder.
Additional Detail
Figure 1. Sample Poodle (A), Giant Schnauzer (B), English Mastiff (C), and Belgian Tervuren pedigrees with seizure transmission. Filled in symbols represent dogs having had seizures.
| A. Poodle | 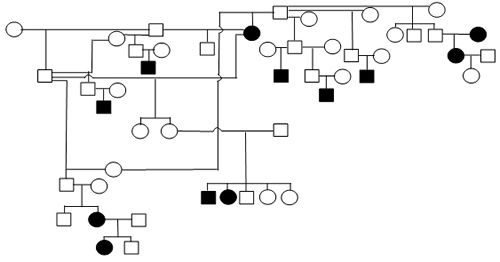
|
|
| |
| B. Giant Schnauzer | 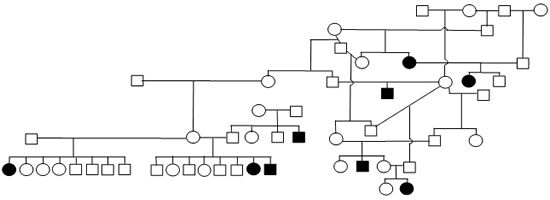
|
|
| |
| C. English Mastiff | 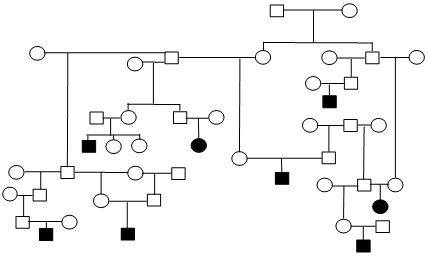
|
|
| |
| D. Belgian Tervuren | 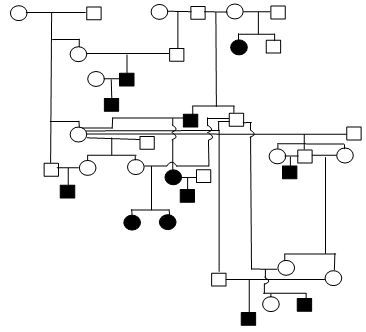
|
|
| |
References
1. Casal ML, Munuve RM, Janis MA, Werner P, Henthorn PS. 2006. Epilepsy in Irish Wolfhounds. J Vet Intern Med. 20:131-5.
2. Chandler K. 2006. Canine epilepsy: what can we learn from human seizure disorders? Vet J. 172:207-17.
3. Ellegren H. 2005 Genomics: the dog has its day. Nature. 438:745-6.
4. Famula TR, Oberbauer AM, Brown KN. 1997. Heritability of epileptic seizures in the Belgian Tervuren. J. Sm. Anim. Prac. 38:349-352.
5. Hall SJ, Wallace ME. 1996. Canine epilepsy: a genetic counselling programme for keeshonds. Vet. Rec. 138:158-160.
6. Ianzano L, Zhang J, Chan EM, Zhao XC, Lohi H, Scherer SW, Minassian BA. 2005. Lafora progressive Myoclonus Epilepsy mutation database-EPM2A and NHLRC1 (EPM2B) genes. Hum Mutat.26:397.
7. Jaggy A, Faissler D, Gaillard C, Srenk P, Graber H. 1998. Genetic aspects of idiopathic epilepsy in Labrador retrievers. J Small Anim Pract. 39:275-80.
8. Licht BG, Licht MH, Harper KM, Lin S, Curtin JJ, Hyson LL, Willard K. 2002. Clinical presentations of naturally occurring canine seizures: similarities to human seizures. Epilepsy Behav. 3:460-470.
9. Lohi H, Young EJ, Fitzmaurice SN, Rusbridge C, Chan EM, Vervoort M, Turnbull J, Zhao XC, Ianzano L, Paterson AD, Sutter NB, Ostrander EA, André C, Shelton GD, Ackerley CA, Scherer SW, Minassian BA. 2005. Expanded repeat in canine epilepsy. Science. 307:81.
10. Patterson EE, Mickelson JR, Da Y, Roberts MC, McVey AS, O'Brien DP, Johnson GS, Armstrong PJ. 2003. Clinical characteristics and inheritance of idiopathic epilepsy in Vizslas. J Vet Intern Med. 17:319-25.
11. Patterson EE, Armstrong PJ, O'Brien DP, Roberts MC, Johnson GS, Mickelson JR. 2005. Clinical description and mode of inheritance of idiopathic epilepsy in English springer spaniels. J Am Vet Med Assoc. 226:54-8.
12. Podell M, 2004. Seizures. In: Platt, S., Olby, N., (Eds.), BSAVA Manual of Canine and Feline Neurology, BSAVA, pp. 97-112.
13. Srenk P, Gaillard A, Busato C, Horin P. 1994. Genetische grundlagen der idiopathischen epilepsie biem Golden Retriever. Teirarztliche Praxis 22:574-578.
14. Sutter NB, Ostrander EA. 2004. Dog star rising: the canine genetic system. Nat Rev Genet. 5:900-10.