Alan H. Rebar1; Rose E. Raskin2, DVM, PhD, DACVP
General Principles of Cytologic Evaluation
It is the primary goal of the cytologist to define a cytologic response as a normal cell population, a malignant neoplastic population, an inflammatory cell population, or a mixed cell population (both inflammatory and neoplastic) (Figure 1). If the reaction is inflammatory an attempt is made to further classify the reaction as to type (for example, neutrophilic versus macrophagic) and to identify etiologic agents. The criteria used to differentiate these various inflammatory cytologic responses are described below.
Cytology of Inflammation (Table 1)
Inflammatory reactions are cytologic responses in which inflammatory cells--neutrophils, eosinophils, lymphocytes, monocytes or macrophages--are the predominant cells seen. Inflammatory reactions may be further classified as neutrophilic, mixed, macrophagic or granulomatous.
Neutrophilic Inflammation
In neutrophilic inflammation, neutrophils account for 70% or more of all inflammatory cells. The remaining cells usually include varying numbers of lymphocytes, eosinophils, and macrophages. Neutrophilic inflammation may have either non-degenerate or degenerate neutrophils. In reactions without degeneration, the neutrophils are unaltered (resemble those in the peripheral blood) or exhibit only the aging change of nuclear hypersegmentation. In contrast, in reactions with degeneration, both neutrophil nuclei and cytoplasm are abnormal. Nuclear changes are those of cellular death--pyknosis, karyorrhexis and karyolysis. Cytoplasmic changes include basophilia and vacuolation.
Neutrophilic inflammation with degenerate neutrophils reflects the action of toxins on infiltrating neutrophils; it is almost invariably associated with bacterial infections. Consequently, whenever degenerate neutrophils are seen in a cytologic specimen, a bacterial etiologic agent should also be sought. In contrast, acute inflammation with non degenerate neutrophils is usually associated with severe irritation of a non-infectious nature. It should be emphasized that this separation of neutrophilic inflammation into subtypes is a generalization and therefore, somewhat artificial; for example, nocardiosis is characterized by neutrophilic inflammation with degenerate neutrophils in areas immediately adjacent to bacterial colonies and neutrophilic response without degenerate neutrophils in areas where no bacteria are seen.
Separating neutrophilic inflammatory responses into degenerative and non-degenerative subtypes may also be of prognostic value. This can be best illustrated by considering the cytologic responses encountered in peritoneal fluids associated with intestinal vascular accidents such as volvulus or intussusception. In these conditions, the principal lesion is infarction of the intestinal wall. Early in the course of the disease process, there is severe irritation of the intestinal wall and an acute irritative peritonitis results. Cytologically, this is reflected as neutrophilic inflammation with non-degenerate neutrophils. As the process continues, however, the lesion becomes more severe and life threatening. Stasis of gut contents generally occurs in the infracted segment. This is often accompanied by bacterial proliferation and toxin production. Additionally, if infarction of the intestinal wall is complete, necrosis of the wall occurs and bacterial toxins and bacteria are leaked into the peritoneal cavity causing acute septic peritonitis. Cytologically, the response is one of neutrophilic inflammation with degenerate neutrophils. In the author's experience the prognosis for surgical correction at this point is considerably more guarded than for the same disease process at a point in time when only non-degenerate neutrophils are observed.
Mixed Inflammation
Mixed inflammatory responses are defined as those responses where 50-70% of the inflammatory cells are neutrophils and the bulk of the remaining inflammatory cells are monocytes and macrophages. Such reactions reflect less severe irritation than neutrophilic inflammatory responses. They may represent a stage in the resolution of a more acute lesion or simply a tissue response to a less irritating etiologic agent than pyogenic bacteria. Systemic mycotic agents such as Histoplasma capsulatum or Blastomycetes dermatitidis commonly elicit mixed inflammatory responses. Since mixed inflammatory responses virtually always reflect less severe irritation than acute responses, they only rarely contain degenerate neutrophils. (It is important to recognize that the degenerate nature of an inflammatory reaction is evaluated strictly on the basis of neutrophil morphology--monocytes and macrophage morphology are not considered.)
Macrophagic Inflammation
Macrophagic (histiocytic) inflammatory responses are those in which over 50% of the inflammatory cells are monocytes and macrophages. These responses imply low-grade irritation and again are commonly seen with systemic mycotic agents or non-infectious foreign bodies. They may also represent the resolution phase of a previously more active reaction.
Granulomatous inflammation represents a specific category of macrophagic inflammation which is occasionally recognized cytologically. Inflammatory giant cells and epithelioid cells are the hallmark of granulomatous inflammation. When granulomatous inflammation is observed, an etiologic agent such as the systemic mycotic agents or a foreign body can be almost certainly identified.
Eosinophilic Inflammation
Eosinophilic exudates (inflammatory responses containing large numbers of eosinophils) represent special inflammatory responses and merit specific consideration. Eosinophils are occasionally seen in large number sin fine needle aspirates from focal inflammatory skin lesions in cats including feline rodent ulcers (eosinophilic granulomas). Aspirates of lick granulomas in dogs may also contain significant numbers of eosinophils. Since mast cell neoplasms may also contain large numbers of eosinophils, care must be taken to distinguish these two conditions in the dog. In horses, fine needle aspirates of cutaneous parasitic lesions (cutaneous habronemiasis or onchocercosis) often contain significant numbers of eosinophils. Microfilariae are occasionally seen in aspirates of onchocercosis.
The author has also seen several eosinophilic exudates involving the pleural or peritoneal cavities in horses and dogs. In dogs, these eosinophilic exudates have been associated with heartworm disease or disseminated mast cell neoplasia. In horses exhibiting acute colic secondary to verminous arteritis, eosinophilic peritoneal exudates are commonly encountered. In general, eosinophilic exudates are most prevalent in parasitic infestations or allergic phenomena.
Table 1. Cytologic classification of inflammation.
|
Inflammatory Classes |
Cell Populations |
Morphologic Subtypes |
Possible Etiologies |
|
Neutrophilic |
>70% neutrophils |
Non-degenerative-neutrophils resemble those of peripheral blood or feature hypersegmented nuclei |
Severe irritant |
|
Degenerate-neutrophils exhibit karyolysis, pyknosis and karyorrhexis; cytoplasmic basophilia and vacuolation eosinophilic--large numbers of eosinophils |
Pyogenic bacteria
Parasites |
|
Mixed |
50-70% neutrophils, |
|
Resolving acute response |
|
30-50% monocytes and macrophages |
|
Irritant of intermediate severity |
|
Macrophagic (Histiocytic) |
>50% macrophages |
Granulomatous-epithelioid cells |
Resolving acute response |
|
Cells and/or giant cells |
Low grade irritant:
foreign body
systemic mycosis |
| Figure 1. General approach to the interpretation of cytologic specimens. | 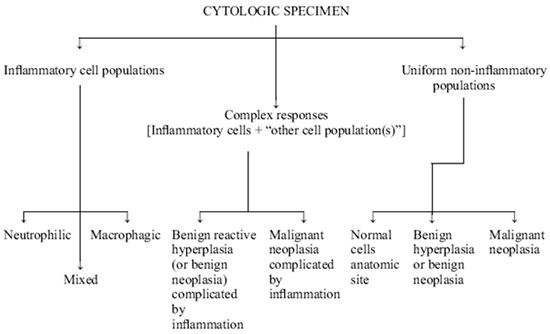
Modified with permission from Current Veterinary Therapy VII, R.W. Kirk, Ed., W.B. Saunders, & Co., Philadelphia, PA 1980. |
|
| |