Gregory K. Ogilvie, DVM, DACVIM (Specialties of Internal Medicine, Oncology)
Director, CVS Angel Care Cancer Center; President, Special Care Foundation for Companion Animals
San Marcos, CA, USA
Adapted from: Ogilvie GK, Moore AS. Managing the Veterinary Cancer Patient. Trenton NJ, Veterinary Learning Systems. 1995, 542 pages; Ogilvie GK, Moore AS. Feline Oncology: A Comprehensive Guide for Compassionate Care. Trenton NJ, Veterinary Learning Systems. 2002, 550 pages and Ogilvie GK, Moore AS. Managing the Canine Cancer Patient: A Practice Manual. Trenton NJ, Veterinary Learning Systems
Feline Cutaneous Squamous-Cell Carcinoma
Incidence, Signalment, and Etiology
Squamous-cell carcinomas occur mostly in adult cats especially around the head and neck, and particularly the ears, nose, and eyelids of cats lacking cutaneous pigment. In these locations the tumor is sunlight-induced in the same manner as described for dogs. Siamese cats appear less likely to develop cutaneous squamous-cell carcinoma than other felidae.(1) In a series of 90 cats with nasal planum SCC, 66 cats (73%) had some white skin or hair color.(2)
Clinical Presentation and History
On the face and ears of cats lacking cutaneous pigment, there is a clear clinical progression of these lesions. Initially the area is erythematous and may have a waxy, dark crust that is easily removed. These lesions appear histologically as either actinic keratosis (precancer) or as carcinoma in situ (non-invasive cancer). Ulceration progresses if the lesion is untreated, with subsequent invasion and destruction of surrounding structures by the tumor.
Staging and Diagnosis
Actinically induced cutaneous squamous-cell carcinoma in cats rarely, if ever, metastasizes. In a series of 90 cats with nasal planum SCC, 6 were found to have metastasis to mandibular lymph nodes and 1 to lungs(2). However, this often occurred late in the course of disease. Regional lymph nodes should be palpated, and fine-needle aspiration or biopsy performed if they are enlarged. Thoracic radiographs are not usually indicated for this tumor in cats. In a study of 90 cats, 17% were found to be in stage T1, 31% as T2, 37% as T3, and 15% as T4.
Prognostic Factors
Tumor proliferative fraction, as measured by immunohistochemical detection of Proliferating Cell Nuclear Antigen (PCNA) was found to be prognostic for control of nasal planum SCC in cats treated by radiotherapy.(2) In addition, tumor stage was found to be prognostic for tumor control. Cats with smaller tumors (T1) had not reached a median survival (i.e., fewer than half of the cats had tumor recurrence) but the average was 53 months while larger tumors (T3) were controlled for a median of 9 months(2).
Treatment
While the synthetic retinoid 13 cis-retinoic acid has not been shown to reverse pre-neoplastic changes for SCC in cats,(3) newer retinoids such as etretinate have not yet been evaluated in this species. In view of the efficacy of etretinate in dogs, it would seem logical that these newer retinoids may also show efficacy for feline preneoplastic squamous-cell carcinoma.
| 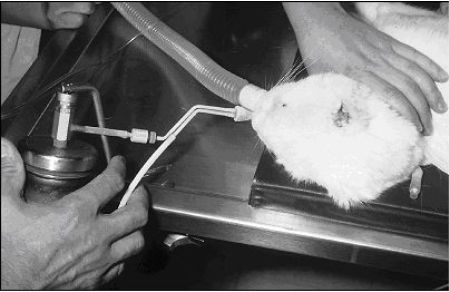
Two rapid freezes and 2 slow thaws with liquid nitrogen cryotherapy with a contact applicator is generally very effective for controlling nasal squamous cell carcinomas less than 1 cm in diameter. |
|
| |
Resection of the pinna for aural SCC is effective in the majority of cats if adequate resection is achieved. Essentially the entire pinna should be removed. However, these tumors may recur locally, as can SCC of the eyelids and nasal planum.
Actinically induced SCC in the cat is very sensitive to radiation therapy. Precancerous plaques and early lesions may be treated with brachytherapy radiation (e.g., strontium-90) at a single high dose. In a group of 25 cats treated with strontium-90, nearly 90% were free of tumor at one year with an average tumor-free period of 34 months.(4)
Local current-field radiation hyperthermia (50oC for 30 seconds) was very effective in causing tumor regression in superficial SCC of cats.(5) Of 19 cats with SCC, 13 (68%) had complete regression. Tumors that did not extend 2 mm or deeper in tissue responded best. Duration of response was observed for only 2 to 6 months.
For more advanced lesions, external-beam teletherapy produces long remissions. It should be remembered, however, that the cat is still susceptible to acquiring new tumors from sunlight exposure, and its behavior should be appropriately modified. Preferably cats should be protected from sunlight exposure during the middle part of the day. Ninety cats with SCC of the nasal planum were treated with orthovoltage-radiation therapy to a dose of 40 Gray in 4-Gray fractions(2). The median control of tumors for these cats was 14 months. However, advanced tumor stage (i.e., larger tumors) affected outcome adversely (see above). Fifteen cats whose tumors recurred were re-irradiated successfully.
Cryotherapy has been recommended as a treatment for SCC of the face in cats. In one study, however, this modality was considerably less effective than either surgery or radiation therapy in achieving local tumor control(6). Eleven of 15 cats had local tumor recurrence within a median of 6 months after cryotherapy. We recently completed a study involving 87 cats with squamous cell carcinomas of the head that were treated with cryotherapy. The median disease free interval was 14 months. Cats that had lesion less than 1 cm in diameter had a much higher probability of having tumors controlled than those with larger tumors. Therefore, we recommend that tumors that are larger than 1 cm in diameter be treated with other procedures other than cryotherapy.
Reminder: Cisplatin can cause severe, fatal pulmonary edema and pleural effusion if administered systemically. Therefore, Photodynamic therapy has been shown to be quite effective in controlling this tumor in cats. In one study, 7 of 11 feline SCCs of the pinna or nasal planum completely resolved using a chloroaluminum sulfonated-phthalocyanine photosensitizer, and 5 of these responses lasted 44 weeks or longer.(7) In another trial using aluminum phthalocyanine tetrasulfonate, long-term (3 to 18 months) responses were seen in 12 of 17 patients(8). However, toxicities, including 1 fatality, were more prevalent in this study. Paradoxically, cisplatin was used successfully in a unique collagen-matrix intralesional implant system to treat 118 feline squamous-cell carcinomas(1). In this study, 83% of cats had a > 50% reduction in tumor volume and 64% had complete resolution after 6 treatments. The lack of systemic toxicity seen in these cats was ascribed to the depot nature of the treatments and subsequent slow release of cisplatin. Sterilized sesame oil appears to function as a similar vehicle to collagen matrix, and may be used at a dose of 2 ml of sesame oil to 10 mg cisplatin in 1 ml of saline.(9)
Mitoxantrone chemotherapy rarely brings objective response in feline oral SCC, but could be considered as an option for metastatic skin lesions.(10) When combined with external-beam radiation therapy, mitoxantrone has been shown to control oral SCC for a median of 170 days, which is substantially longer than when either modality is used alone.(11) Three cats with dermal SCC showed partial responses of short duration to bleomycin chemotherapy.(12) Carboplatin is a new cisplatin-like compound that may be of value for treating metastatic squamous cell carcinoma. Alan Theon recently reported that when a carboplatin/sesame seed oil compound was used to treat cats with squamous cell carcinoma, the results were excellent.
| 
If cryotherapy or intralesional chemotherapy is not indicated, a nosectomy procedure is well accepted by most cats and their owners for the treatment of squamous cell carcinoma of the nasal planum. |
|
| |
Oral Squamous-Cell Carcinoma in Cats
In contrast to the situation in dogs, oral SCC in cats is a highly aggressive disease that responds poorly to surgical treatment or to radiation therapy regardless of its location in the mouth. The longest control and survival rates have been obtained using a combination of radiation therapy and mitoxantrone chemotherapy.(11)
The biggest problem for cats that have oral squamous cell carcinomas is that they do not maintain an adequate plane of nutrition. Appetite stimulants and very aromatic foods should be used whenever possible. Care should be taken when feeding baby foods because they often contain onion powder that can cause heinz body anemia.
Carboplatin chemotherapy with or without radiation therapy appears to be resulting in good efficacy. The single most important prognostic feature appears to be bone involvement.
| 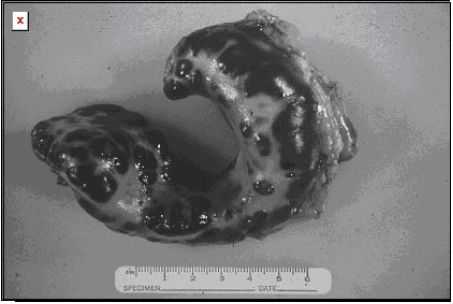
Splenic mast cell tumors differ in the cat from the dog in that if it is just localized to this structure, resection can result in survival that exceeds 1 year. |
|
| |
Hemangioma-Hemangiosarcoma In Cats
Incidence, Signalment, and Etiology
Cutaneous hemangiomas and hemangiosarcomas are rare in cats, and occur mainly in older animals. This tumor is similar to squamous-cell carcinoma in that it is actinic, or sunlight-induced. Cutaneous hemangiosarcoma occurs commonly in cats from areas where actinically induced tumors are common(18) (California, Missouri, Florida), but not in cats from the northeastern United States.(18,19,20)
Clinical Presentation and History
Feline hemangiomas appear as solitary tumors in the dermis and subcutis without any site predilection. In contrast, feline hemangiosarcomas may have a predilection for the head (ear tips, nasal planum) and also have a predilection for non-pigmented skin.(17) They are usually solitary.
Treatment
Surgical excision of cutaneous hemangioma usually has a good prognosis, although local recurrence following surgical excision of cutaneous hemangiosarcoma is frequent. Metastasis appears to be rare. It is therefore appropriate to treat this tumor as a soft-tissue sarcoma in cats, with aggressive initial surgery as the best therapeutic approach.
Round (Discrete) Cell Tumors
This group of tumors may equally be called discrete cell tumors, and their appearance on cytologic preparations is that of clumps or individual cells that are round in appearance without obvious attachment to other cells. Round cell tumors include mast cell tumors, histiocytoma, lymphoma, plasmacytoma and transmissible venereal tumor.
Mast-Cell Tumors
Introduction
Masses composed of mast cells can be either reactive or neoplastic, and this can pose certain problems in nomenclature. Mast-cell tumors are a very common neoplasm of the cat. Mast cells contain dark-staining granules that contain histamine, heparin, and other vasoactive amines. These characteristic granules make recognition of mast-cell tumors on cytology relatively simple. For further information please refer to the Mast Cell Tumor section.
Mast-Cell Tumors in Cats
Incidence, Signalment, and Etiology
Mast-cell tumors were the only cutaneous tumor diagnosed in cats younger than one year in one study.(1) In the same study, Siamese cats were three times more likely to develop cutaneous mast-cell tumors. Tumors in Siamese cats are usually subcutaneous and composed of "histiocytic" cells. These tumors may regress without therapy.(24)
| 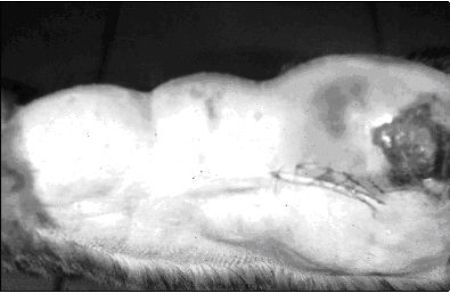
Generalized mammary gland enlargement in a young cat that is not spayed may quite likely be fibroepithelial hyperplasia. These spontaneously resolve. |
|
| |
Clinical Presentation and History
The most common cutaneous mast cell tumors in cats are single, firm, and circumscribed dermal nodules.(25) Appearance of multiple similar masses is the next most common presentation. These tumors are usually histologically well-differentiated and have a benign clinical course.(2) While most cutaneous MCTs in the cat are benign, in some studies, cutaneous tumors are associated with malignant disease evidenced by visceral involvement.(25,26) Lymphoreticular mast-cell tumors are often seen in cats, with marked splenomegaly the most common finding. Diffuse cutaneous disease may occur with this form of MCT. Mastocythemia and bone-marrow involvement are seen in many of these cats, and the presenting signs of vomiting and anorexia are presumably owing to tumor degranulation. Intestinal mast-cell tumors have been described in cats; they are always malignant.
Staging and Diagnosis
The suspicion of MCT in a chronically vomiting cat with marked splenomegaly can be confirmed by fine-needle aspirate of the spleen. Less frequent sites for lymphoreticular mast-cell tumors in cats are the mediastinum (with resultant pleural effusion) and lymph nodes. These cats often have high numbers of circulating mast cells as well as anemia and other cytopenias from bone-marrow infiltration and erythrophagocytosis by malignant cells.
Treatment
The treatment of choice for cutaneous MCT in cats is surgery; for solitary tumors, a good prognosis can usually be given. Some cats may develop multiple well-differentiated tumors, and these cats may be treated with multiple palliative surgeries or corticosteroids (1 mg/kg prednisone daily). For invasive or incompletely excised MCTs, radiation therapy appears to be a successful adjunct to surgery; however, data regarding tumor control and patient survival are not as well established as for dogs.
Mitoxantrone caused a partial response in a feline mast-cell tumor, but the drug has not been further evaluated for the treatment of this disease.(10)
The treatment of choice for lymphoreticular MCTs in cats is splenectomy; long-term survival occurs in many cats receiving no other therapy. Response to splenectomy seems greater than would be explained by simple tumor mass reduction, as hematologic and other organ involvement apparently resolve. It is possible that splenic suppressor-cell activity may be reduced after splenectomy allowing for some control by the cat's immune system. For this reason the use of postoperative corticosteroids in these cats in controversial.(27.28)
Mammary Adenocarcinoma
Background
Owners frequently present their cat with mammary mass for a "skin condition on the underside". When a cat with a mammary mass is presented, a malignancy must be considered. At least 70-90% of feline mammary tumors are malignant. Mammary tumors are known to be at least the third most frequently occurring tumor in the cat, following hematopoietic neoplasms and skin tumors. The incidence of mammary tumors in the cat is less than half that of dogs. Although there is no proven breed-associated predilection for mammary tumors, some investigators have suggested that domestic short-haired and Siamese cats have higher incidence rates than other cats. Siamese cats may have twice the risk of any other breed of developing mammary tumors.
Mammary neoplasia has been reported to occur in cats from nine months to 23 years of age, with a mean age of occurrence of 10 to 12 years. One study suggests that the disease occurs at an earlier age in Siamese cats and the incidence reaches a plateau at about nine years of age. The majority of affected cats are intact females, however the disease is occasionally seen in spayed females and rarely in male cats. More than 80% of the feline mammary tumors are histologically classified as adenocarcinomas. The frequency of diagnosis of the specific types of adenocarcinomas differs slightly among pathologists, but most agree that tubular, papillary, and solid carcinomas are the most common. The majority of the adenocarcinomas have a combination of tissue types in each tumor. Sarcomas, mucinous carcinomas, duct papillomas, adenosquamous carcinomas and adenomas are rarely seen.
Clinical Presentation and History
Cats with mammary tumors are often presented to the veterinarian five months after they were initially noted. Thus, the tumors are usually in an advanced state of development when they are handled clinically. The mammary tumor often appears as a locally invasive mass that has metastasized frequently. The neoplasm may adhere to the overlying skin but rarely is adhered to the underlying abdominal wall. The tumor is usually firm and nodular. At least one-quarter of affected patients have ulcerated masses.
The infiltration of lymphatics may be clinically apparent as subcutaneous linear, beaded chains. Swelling due to tumor thrombi or decreased vascular return can cause discomfort, edema, and a change in the temperature in the pelvic limbs. The involved nipples are red and swollen and may exudate a tan or yellow fluid. The tumor can involve any or all mammary glands and is noted equally in the left and right sides. A slightly higher incidence has been noted in the cranial two glands by some investigators. More than half of the affected cats have multiple-gland involvement. These tumors can be associated with chronic mastitis, uterine disease, and other unrelated tumors, as well as anemia, osteoporosis, ascites, and leukocytosis.
| 
Radical mastectomy of the entire chain, not regional lumpectomy is essential for local control for cats with mammary adenocarcinoma. |
|
| |
Staging and Diagnosis
Before any diagnostic or therapeutic steps are taken, the health status of the cat must be fully assessed. A chemical screen, urinalysis, and a complete blood count should be done to identify any presurgical abnormalities. In several studies, more than 80% of the cats with a mammary malignancy had metastases to one or more of the following organs at the time of euthanasia; lymph nodes, lungs, pleura, liver, diaphragm, adrenal glands, and kidneys.(31-33) Thoracic radiographs in both the right and left lateral and ventrodorsal planes should be made to search for pulmonary, lymph node, and pleural metastases. Mammary tumor pulmonary metastases appear radiographically as interstitial densities. They range from those that are faintly seen, to those that are several centimeters in diameter, to miliary pleural lesions that can produce significant effusion. Sternal lymphadenopathy is occasionally seen. Whenever regional lymph nodes are evaluable, they should be assessed by fine needle aspiration cytology or biopsy. Aging changes in the lungs and pleura as well as inactive inflammatory lesions may stimulate metastatic disease. Treatment should not be withheld because of equivocal radiographic findings.
Because of the high frequency of malignancy, an aggressive approach should be taken to confirm the diagnosis. A preliminary biopsy is not recommended unless it will change the owner's willingness to treat or the surgical procedure. Tissue for histopathology is taken at the time of mastectomy and should include the regional lymph nodes. If pleural fluid is removed from a cat with a mammary gland lesion, cytology should be done on the fluid to search for malignant cells.
A variety of nonmalignant lesions must be considered in a differential diagnosis of mammary neoplasia. The most common benign growths are classified as cysts, papillary cystic hyperplasia, lobular hyperplasia, and mastitis. Fibroepithelial hyperplasia is a common benign lesion involving one or more glands and is frequently seen one to two weeks following estrus. The gland may be so large that the patient may walk with an abnormal gait and the skin overlying the mass may be discolored, *edematous, and painful. Cats with fibroepithelial hyperplasia are often young, intact females. The signs are similar to those seen with most malignant tumors. Because the benign masses closely resemble malignant neoplasms, they are often treated as malignancies.
Prognostic Factors
In the last 20 years, little progress has been made in extending the survival time of canine and feline mammary tumor patients. Because stromal invasion is almost always present and metastases are frequently present at the time of surgery, a guarded to poor prognosis should always be given. Sixty-six percent of the cats that have had their tumors surgically excised have a recurrence at the surgical site. Most studies state that the time from tumor detection to the death of the cat is rarely over 12 months. The most significant prognostic factors influencing tumor recurrence and survival for cats with malignant mammary neoplasia are tumor size, the extent of surgery needed to remove the tumors, and histologic grading of the tumors.
MacEwen has shown that tumor size is the most important of these prognostic factors. Following surgery, the median for survival for cats with tumors > 3 cm in diameter is 6 months; for cats with tumors 2 to 3 cm in diameter, the median for survival following surgery is 2 years; and for cats with tumors < 2 cm in diameter, the median for survival after surgery is approximately 3 years. Radical surgery, when compared with regional "lumpectomy", has been shown to reduce local tumor recurrence but not to increase the overall time of survival. Cats with well-differentiated tumors with few mitotic figures per high-power field live longer compared with those with tumors that are not as well-differentiated histopathologically. The one-year survival rate was high in cats with a tumor that did not show lymphatic infiltration. There is a good correlation between the grade of malignancy, method of growth, and prognosis. Patients with pulmonary metastatic disease rarely survive longer than two months.
Mammary neoplasms in the cat have been treated in a variety of ways however, surgery is the most widely used treatment. There have been no reports documenting the efficacy of radiation therapy or commercially available biological response modifiers for the treatment of this disease. The biological response modifier, liposome-encapsulated muramyltripeptide-phosphatidyethanolamine (L-MTP-PE) may not be effective when used in combination with surgery or chemotherapy.
The success of surgery is hindered by the invasive nature of the disease and its tendency for early metastasis. Radical mastectomy (i.e., removal of all glands on the affected side) is the surgical method of choice because it significantly reduces the change of local tumor recurrence. This procedure is frequently utilized regardless of the size of the tumor.
Several surgical principles are observed when performing a mastectomy on feline mammary tumor patients. During or prior to surgery, a bacterial culture and antibiotic sensitivity testing may be indicated because of approximately one-fourth of mammary carcinomas are ulcerated. An en bloc resection is often employed in such a way that the tumor and draining nodes and vessels are removed by wide surgical excision and a partial or complete resection of the underlying tissue is done. If bilateral mastectomy is indicated, the affected glands and their associated lymph nodes are removed and a second surgery is performed 10 to 14 days later, the interim allows the skin to stretch for a complete closure at the second surgery. Early vessel ligation is essential; one study noted that two-thirds of the cases examined had tumor invasion of lymphatics and veins. Gentle handling of all damaged tissue is essential. Copious flushing of the surgery area after the tumor is removed helps eliminate exfoliated neoplastic cells. Although spaying has been shown not to decrease the incidence of recurrence, some believe that it is warranted because of the occasionally seen coexisting ovarian and uterine disease.(33) If the mammary mass is due to a benign condition such as fibroepithelial hyperplasia, spaying often results in regression of the hyperplastic tissue. Regression may take up to five months. This condition often resolves spontaneously within a few weeks of diagnosis; in some cases without performing an oophorectomy.
| 
Very large resections, often including the dorsal spinous processes is needed to completely remove vaccine associated sarcomas of the intrascapular region. |
|
| |
Radiation Therapy
Radiation therapy is not used routinely to treat feline mammary tumors. Presently, there are no major claims that radiation dramatically increases the survival rate of feline mammary tumor patients, however it may reduce local recurrence rates.
Chemotherapy
Chemotherapy, alone or in combination with surgery, is not as successful for feline mammary tumors as it is for other feline tumors such as lymphoma. Cyclophosphamide has been used alone or in combination with other chemotherapeutic agents and has not consistently helped the feline mammary tumor patient. The combination therapy of doxorubicin and cyclophosphamide has been shown to induce short-term partial and complete responses in 50% of cats with metastatic or nonresectable local disease. This chemotherapeutic protocol has been shown to be toxic in some cats and does not prolong survival. Other drugs that may be used include mitoxantrone and taxol. The role of the later two drugs is still being elucidated, however both have some efficacy in cats with malignant neoplasia. Further studies are necessary to quantify the benefit of adjunctive therapy for mammary neoplasia in cats. Chemotherapy should be used by practitioners that are familiar with the use of these drugs and their side effects.
Vaccine Associated Sarcomas
Recent epidemiologic studies have associated the administration of a number of vaccines and the development of soft tissue sarcomas in the cat. Vaccines have also been associated with hematological or immunological diseases. Perhaps the most disconcerting problem is the vaccine associated malignancy problem. In a recent epidemiologic study,(34,35) sarcomas were temporally associated with previous injection of various vaccines into specific body locations. Feline leukemia virus, rabies vaccination, and development of fibrosarcomas at the injection site within a year following vaccination were statistically associated. This study demonstrated a 5.5% increased risk of developing sarcomas in response to feline leukemia virus vaccination and a twofold increase in risk of development of sarcomas after rabies vaccination. The actual incidence of the tumor was estimated to be approximately one sarcoma per 10,000 feline-leukemia virus and rabies vaccines administered. No association between sex, breed, and concurrent viral infections in the development of these sarcomas was found. The aluminum adjuvant in many of these vaccines may be associated with development of vaccine-associated sarcomas.(35-37) However, Kass, et al.,(35) demonstrated that certain aluminum-free vaccines may also be associated with development of these soft-tissue sarcomas. Increase in development of soft-tissue sarcomas following one vaccination was 50%, following two vaccinations was 127%, and following three or four vaccines simultaneously administered at the same location was approximately 175%.(35) Therefore, there appears to be a multifactorial association between vaccines and growth of sarcomas.(34,37) The development of sarcomas in response to vaccines, especially those that contain aluminum as an adjuvant, may suggest an association between foreign bodies and the growth of these neoplasms. This is not a new observation. Indeed, other metals such as arsenic, chromium, and nickel have been shown under an array of conditions to induce sarcoma formation.3,8 Histologically, vaccine-associated sarcomas are enveloped in dense, fibrous connective tissue and infiltrated with inflammatory lymphocytes and macrophages. Macrophages often obtain bluish-gray foreign material that electron-probe x-ray microanalysis identifies as aluminum and oxygen.(35,36) Other tumors develop in apparent association with other foreign bodies. For example, ocular sarcomas have been associated with foreign material in the eye.(38) In addition, osteosarcomas have been associated with metallic implants. Despite the compelling information, no specific etiology has been determined for the development of these sarcomas, only causal association. Additional research must answer the question of etiopathogenesis. Vaccine associated soft-tissue sarcomas are frequently located in such areas of previous vaccination as between the shoulder blades and in the hind leg. A diagnosis is made by fine-needle aspirate cytology or, preferably, incisional biopsy. The clinician should keep in mind that these soft-tissue sarcomas are encased in a pseudocapsule that is actually compressed tumor tissue. In addition, tendrils of tumor extend far beyond the site of palpable tumor.
Radiation is very important for the treatment of cats with vaccine associated sarcomas. This may be given before or after surgery and is given along with chemotherapy in most cases.
Treatment must be aggressive regardless of tumor size. These soft-tissue sarcomas are extremely aggressive and extend far beyond the palpable site. While the most effective treatment is not known, surgery should be employed whenever possible. In each case, extremely wide and deep surgical margins (> 3 cm), including all the soft-tissue structures and, if appropriate, any bony structures in the region of the postvaccinal sarcoma should be obtained. Essentially, the tumor and a large "cuff" of normal tissue surrounding the mass should be removed en bloc. All lateral and deep margins should be marked and submitted for histopathology after appropriate formalin fixation to determine presence of residual neoplastic disease. In some veterinary oncology centers, presurgical radiation therapy is routine to minimize the amount of viable tumor around the palpable tumor. If recurrence is noted or suspected after surgical removal of the tumor, radiation therapy can be directed widely around the surgery site. In addition, doxorubicin may be effective for controlling local disease for a period of time. In some centers, surgery, radiation therapy, and chemotherapy are used in conjunction to control this tumor. Until more effective treatments are known, the following recommendations may help prevent development of postvaccinal sarcomas:
1. Avoid administering multiple vaccines in the same site;
2. Administer vaccines in extremities (Rabies Right; Leukemia Left, FVRCP in the right shoulder) that may allow amputation as an effective treatment;
3. Remove postvaccinal granulomas that persist several months after vaccination; and
4. Continue research on different adjuvants to reduce the development of vaccine-induced sarcomas.
Finally, many have questioned the wisdom of administering vaccines on a yearly basis, especially when using multivalent products.
References
1. Miller MA, Nelson SL, Turk JR, Pace LW, Brown TP, Shaw DP, Fischer JR, Gosser HR. Cutaneous neoplasia in 340 cats. Vet Pathol 28: 389-395, 1991.
2. Brooks MB, Matus RE, Leifer CE, Alfierir AA, Patnaik AK. Chemotherapy versus chemotherapy plus radiotherapy in the treatment of tonsillar squamous cell carcinoma in the dog. J Vet Internal Med 2: 206-211, 1988.
3. Theon AP, Madewell BR, Shearn V, Moulton JE. Irradiation of squamous cell carcinomas of the nasal planum in 90 cats. Proc 13th Annual Conf Veterinary Cancer Soc. 147-148, 1993.
Evans EG, Madewell BR, Stannard AA. A trial of 13-cis-retinoic acid for treatment of squamous cell carcinoma and preneoplastic lesions of the head in cats. Am J Vet Res 46: 2553-2557, 1985.
4. VanVechten MK, Theon AP. Strontium-90 plesiotherapy for treatment of early squamous cell carcinomas of the nasal planum in 25 cats. Proc 13th Annual Conferinary Cancer Soc 107-108, 1993.
5. Grier RL, Brewer WG, Jr., Theilen GH. Hyperthermia treatment of superficial tumors in cats and dogs. J Am Vet Med Assoc 177: 227-233, 1980.
6. Atwater SW, Powers BE, Straw RC, Withrow SJ. Squamous cell carcinoma of the pinna and nasal planum. Fifty-four cats 91980-1991). Proc Vet Cancer Soc 11th Annual Conf 1991, 35-36.
7. Roberts WG, Klein MK, Loomis M, al et. Photodynamic therapy of spontaneous cancers in felines, canines, and snakes with chloro-aluminum sulfonated phthalocyamine. J Natl Cancer Inst 83: 18-23, 1993.
8. Peaston AE, Leach MW, Higgins RJ. Photodynamic therapy for nasal and aural squamous cell carcinoma in the cat. J Am Vet Med Assoc 202: 1261-1265, 1993.
9. Theon AP, Pascoe JR, Carlson GP, Krag DN. Intratumoral chemotherapy with cisplatin in oily emulsion in horses. J Am Vet Med Assoc 202: 261-266, 1993.
10. Ogilvie GK, Moore AS, Obradovich JE, Elmslie RE, al et. Toxicoses and efficacy associated with the administration of mitoxantrone to cats with malignant tumors. J Am Vet Med Assoc, 202: 1839-1844, 1993.
11. LaRue SM, Vail DM, Ogilvie GK, al et. Shrinking-field radiation therapy in combination with mitoxantrone chemotherapy for the treatment of oral squamous cell carcinoma in the cat. Proc. Eleventh Annual Conference of the Veterinary. Cancer Society, Minneapolis, MN Oct. 27-29, 1991. 99,
12. Kalaher KM, Anderson WI, Scott DW. Neoplasms of the apocrine sweat glands in 44 dogs and 10 cats. Vet Record 127: 400-403, 1990.
13. London CA, Dubielzig RR, Ogilvie GK, al et. Ear canal tumors of dogs and cats: preliminary results of a VCOG retrospective study. Proc Vet Cancer Soc 13th Annual Conf, 1993, 59-60. ,
14. Theon AP, Barthez PY, Madewell BR, Griffey S. Radiation therapy of ceruminous gland carcinomas in dogs and cats. J Am Vet Med Assoc, in press ,
15. Marino DJ, MacDonald JM, Matthiesen DT, Patnaik AK. Results of surgery in cats with cervminous gland adenocarcinoma. J Am Anim Hosp Assoc 30: 54-58, 1994.
16. Little CJL, Pearson GR, Hurvitz AI. Neoplasia involving the middle ear cavity of dogs. Vet Record 124: 54-57, 1989.
17. Hahn KA. Vincristine sulfate as single-agent chemotherapy in a dog and a cat with malignant neoplasms. J Am Vet Med Assoc 197: 796-798, 1990.
18. Miller MA, Ramos JA, Kreager JM. Cutaneous vascular neoplasia in 15 cats: Clinical, morphologic and immunohistochemical studies. Vet Pathol 29: 329-336, 1992.
19. Scavelli TD, Patnaik AK, Mehlaff CJ, Hayes AA. Hemangiosarcoma in the cat: retrospective evaluation of 31 surgical cases. J Am Vet Med Assoc 187: 817-819, 1985.
20. Carpenter JL, Andrews LK, Holzworth J. Tumors and tumor-like lesions. In: Diseases of the cat: Medicine and Surgery, ed Holzworth J, WB Saunders Co, Philadelphia, PA, pp. 406-596, 1987.
21. Lawler DF, Evans RH. Multiple hepatic cavernous lymphangioma in an aged male cat. J Comp Path 109: 83-87, 1993.
22. Stobie D, Carpenter JL. Lymphoangiosarcoma of the mediastinum, mesentery and omentum in a cat with chylothorax. J Am Anim Hosp Assoc 29: 78-80, 1993.
23. Aronsohn MG, Carpenter JL. Distal extremity melanocytic nevi and malignant melanomas in dogs. J Am Anim Hosp Assoc 26:605-612, 1990.
24. Wilcock BP, Yager JA, Zink MC. The morphology and behavior of feline cutaneous mastocytomas. Vet Pathol 23: 320-324, 1986.
25. Scott DW. Feline dermatology 1900-1978: a monograph. J Am Anim Hosp Assoc 16: 331-459, 1980.
26. Garner FM, Lingeman CH. Mast cell neoplasms of the domestic cat. Path Vet 7: 517-530, 1970.
27. Liska WD, MacEwen EG, Zaki FA, Gavery M. Feline sytemic mastocytosis: a review and results of splenectomy in seven cases. J Am Anim Hosp 15: 589-597, 1979.
28. Andrews LK. Tumors and tumor-like lesions: mast cell tumors. In: Diseases of the cat (J. Holzworth, ed) WB Saunders Co, Philadelphia, PA 569-579, 1987.
29. . Moore PF, A Rosin. Malignant histiocytosis of Bernese Mountain Dogs. Vet Pathol 23: 1-10, 1986.
30. Schmidt ML, Rutteman G, Wolvekamp P. Canine malignant histiocytosis (MH): Clinical and radiographic fundings. Tijdschrift voor Diergeneeskunde 117(suppl 1): 43-44, 1992.
31. Ogilvie GK, Moore AS. Mammary Neoplasia. Managing the Veterinary Cancer Patient. trenton NJ, Veterinary Learning Systems. 1995, 430-440.
32. Ogilvie GK, Moore AS. Mitoxantrone chemotherapy in veterinary medicine. In: Current Veterinary Therapy XI. Kirk RW (ed), WB Saunders, Philadelphia, pp 399-401, 1992.
33. Ogilvie GK: Principles of Oncology. In Handbook of Small Animal Internal Medicine. Morgan RV (ed) Churchill & Livingston, Philadelphia, 1992, 799-812.
34. Ogilvie GK. Feline Mammary Neoplasia. In, Feline Medicine and Surgery, Veterinary Learning Systems, Trenton, NJ, 1994:74-83.
35. Hendrick MJ, Kass PH, McGill D, Tivard IR. Postvaccinal sarcomas in cats. J Natl Canc Inst 86:341-335, 1994.
36. Kass PH, Barnes WG, Spangler WL, et al. Epidemiologic evidence for a causal relation between vaccination and fibrosarcoma tumorigenesis in cats. J Am Vet Med Assoc 203:396-405, 1993.
37. Esplin DG, McGill LD, Meninger AG, et al. Postvaccinal sarcomas in cats. J Am Vet Med Assoc 202(8):1245-1247, 1993.
38. Hendrick MJ, Brooks JJ. Postvaccinal sarcomas in the cat: histology and immunohistochemistry. Vet Pathol 31:126-129, 1994.
39. Macy DM. Vaccine-associated sarcomas. Proceedings 12th Annual Veterinary Medical Forum - ACVIM. San Francisco, CA pp 854-856, 1994.
40. Sinibaldi K, Rosen H, Liu S, et al. Tumors associated with metallic implants in animals. Clin Orthop Rel Res 118:257-266, 1976.