Abstract
Mercury Toxicity
Mercury (Hg) has been recognized as a pollutant for thousands of years. However, man has used Hg in increasing amounts in the manufacture of
electrical equipment, scientific instruments, explosives, chemicals, in the electrolytic production of chlorine and alkali, and in agriculture as fungicides and
seed disinfectants.13 The health risks of ingesting Hg-contaminated food first became internationally known after the poisoning outbreak in Japan,
known as Minamata disease. The initial outbreak of this neurological disease occurred after a chemical factory discharged large quantities of mercury directly
into the enclosed marine Minamata Bay. Over a 19 year period, 899 human cases of Minamata disease were officially recognized, with 143 deaths. After numerous
epidemiological studies, Minamata disease was determined to be the result of very high concentrations of Hg in fish and shellfish.2 A second outbreak
occurred in Japan in 1964, along the Agano River. This outbreak consisted of 724 recognized human cases, with 55 deaths.2
From 1940 to 1966, the highly toxic alkyl Hg compounds were widely used as fungicides in the agricultural industries in Sweden and North
America. Bird populations consuming seeds declined and were found to have very high levels of Hg. In 1966, the use of alkyl Hg was banned and the high levels of
Hg in birds were no longer detectable.11 Human populations were also affected by the use of organic Hg compounds. In Iraq, in 1972 and 1973, 6530
people were hospitalized, with 459 deaths, after wheat seed treated with organic Hg fungicides was ingested as bread.11
Since the mid 1970's, acute Hg toxicity has been extensively investigated.4,5,10,13,15 Toxic levels of mercury have been found to
effect multiple organ systems including renal, cardiovascular, reproductive, hepatic, developmental, immune, and gastrointestinal. In addition, acute Hg toxicity
may lead to neoplasia and death. However, the critical organ in Hg toxicity, especially methylmercury (MeHg) toxicity, is the nervous system, in which high levels
of MeHg exposure produce widespread loss of neurons and gliosis in the human and rodent cerebral cortex, cerebellum, and midbrain.11
During the last decade, mercury pollution in the aquatic environment has become of increasing concern because fish have been found to have
very high concentrations of Hg. Mercury and its compounds have been included in the "Black List" of international conventions such as the Oslo, Paris, and
Barcelona Conventions and the EEC Directive on the discharge of dangerous substances, whose aim is to prevent aquatic pollution.15 However, the high
levels of Hg found in fish today are not only linked to individual industrial discharges of mercury, but also to natural degassing of the earth's crust and
particularly widespread air pollution. In the 1980's, it was recognized that mercury is transported long distances in the atmosphere and may be deposited 1,000 to
2,000 km from the original source.9
The majority of Hg released from natural, industrial, and agricultural sources is in the inorganic form. This Hg enters the food chain through
microorganisms, in fresh and marine sediments, which have the ability to methylate Hg.16 Methylmercury (MeHg) is more toxic and more readily
bioaccumulated than inorganic Hg. The Hg in fish occurs almost entirely as MeHg, in fact 99% of Hg in the muscle is in the form of MeHg.1 This highly
toxic MeHg moves up the food chain to marine mammals and humans. To date, no function has been discovered for Hg in living organisms.4
Mercury in the Gulf of Mexico
The Texas coast is a large industrial and agricultural region. Houston and Corpus Christi are two of the ten busiest ports in the United
States, and the concentration of petrochemical industries on the Texas Bay shores is the largest in the country.3 Mercury has been designated as a
"toxic chemical of concern in the marine environment" by the National Marine Pollution Program.12 This program's definition of the term "toxic" is
"material capable of producing an adverse response (effect) in a biological system, seriously injuring structure or function or producing death."
From 1991 to 1996, our laboratory performed complete detailed necropsies on 50 stranded Atlantic bottlenose dolphins (Tursiops
truncatus) ranging in age from neonate to approximately 38 years. Complete necropsies were performed including gross examination and microbiological,
histological, and toxicological sampling of all organs and tissues. We used inductively coupled plasma-mass spectrometry (ICP-MS) to determine the concentrations
of selenium (Se) and Hg present in the liver, kidney, muscle, blubber, and lymph nodes. Our investigations indicated that these dolphins had extremely high levels
of total Hg (up to 864 µg/g dry weight and 1040 µg/g dehydrated wet weight mercury in liver and lymph nodes, respectively), levels that are potentially
lethal in humans and cattle (Figure 1).14 Furthermore, these dolphins did not have significant neuropathology. Thus, we hypothesized that dolphins have
unique mechanisms for tolerating persistently high levels of mercury which are known to be toxic in other mammals.
| Figure 1. | 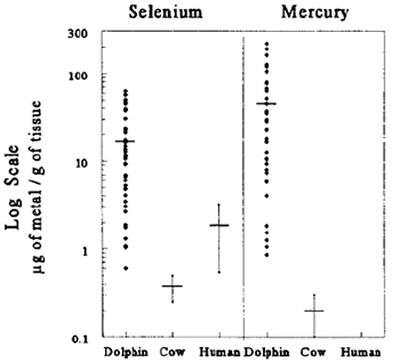
|
|
| |
Adaptive Mechanisms to Tolerate High Levels of Mercury
Figure 2 depicts the possible adaptive mechanisms that we are currently assessing or plan to assess.
Mercuric selenide: Formation of a complex between Hg and Se is a suggested mechanism by which dolphins could tolerate high levels of
Hg.6,8 An equimolar Hg:Se ratio has been documented in most mammals including some cetaceans.7 In our studies, Hg was found in extremely
high levels in the liver and these levels strongly correlated (r2 = 0.985) with hepatic levels of Se.
| Figure 2. | 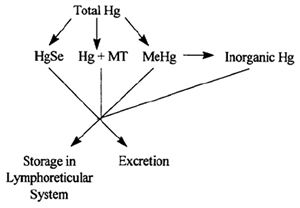
|
|
| |
Storage in Lymphoreticular System: A large majority of the dolphins in our study have pigmented deposits in the liver and
various lymph nodes. We used histopathology procedures to remove melanin pigment, formalin pigment, and mercury precipitated pigment from these tissues. The
pigmented deposits disappeared only after the tissues were treated with mercury bleach. In order to confirm that these deposits contained Hg, we used cold vapor
atomic absorption spectrophotometry (CVAA) and energy dispersive x-ray analysis (EDAX). The deposits contained not only extremely high levels of Hg, but also Se.
Metallothionein: Metallothionein (MT) is a low-molecular-weight, metal binding protein which is thought to play a role in the
absorption, tissue uptake, transport, storage, and detoxification of metals5 in fish, aquatic invertebrates, and mammals. MT has a high content of
conserved cysteine residues which confer a high affinity for metals such as Hg. Therefore, MT may be one means by which dolphins detoxify the high levels of Hg
they accumulate. We have identified associations between MT immunoreactivity and tissue levels of Hg. We immunohistochemically characterized MT presence using a
monoclonal mouse anti-MT antibody (Dako-MT, E9) in the liver and lymph nodes from a group of immature dolphins, and a group of mature dolphins with high levels of
Hg versus a group of similar age dolphins with low Hg levels.
MeHg: Seals and whales are reported to demethylate and store the majority of their Hg burden in an inorganic form.4,10
However, higher levels of MeHg have been noted in animals which were ill, suggesting that weakened or compromised animals may not be able to detoxify MeHg as
efficiently as healthy animals.10 We have already determined that levels of total Hg from the dolphins in this project range from 0.18 to 217.76
µg/g wet weight (864 µg/g dry weight). Future investigations will examine the disposition of Hg in dolphins by comparing the concentrations of organic
(methyl) and inorganic Hg in the various tissues.
Acknowledgments
We thank Elsa M. Haubold and Lance S. Clark for laboratory assistance and Jason P. Turner for teeth aging some of the dolphins in this
study. This work was supported by grant #NA16RGO457-01 from the National Oceanic and Atmospheric Administration (NOAA) through the National Sea Grant College
Program, by grant #MX822147-01-0 from the Environmental Protection Agency (EPA) Gulf of Mexico Program, grant #T35 ES07294 from the National Institutes of
Environmental Health Sciences Toxicology Training Grant, and by the University of Texas Medical Branch Centennial Center in Environmental Toxicology.
References
1. Bloom NS. 1992. On the chemical form of mercury in edible fish and marine invertebrate tissue. Canadian Journal of Fisheries
and Aquatic Sciences. 49:1010-1017.
2. Boudou A, F Ribeyre. 1997. Mercury in the food web: accumulation and transfer mechanisms. Metal Ions in Biological Systems
34:289-319.
3. Center for Marine Conservation. 1992. Environmental quality in the Gulf of Mexico; A citizens guide, second edition.
Center for Marine Conservation, Washington, DC.
4. Clarkson TW. 1994. The toxicology of mercury and its compounds. Pages 631-641 in C.J. Watras and J.W. Huckabee, eds. Mercury
Pollution, Integration and Synthesis. CRC Press Inc., Salem, MA.
5. Gerstner HB, JE Huff. 1977. Clinical toxicology of mercury. Journal of Toxicology and Environmental Health 2:491-526.
6. Fishbein L. 1991. Selenium. In E. Merian, ed. Metals and their Compounds in the Environment. VCH Publishers, Weinheim,
Germany.
7. Koeman JH, WSM van de Ven, JJM de Goeij, PS Tjioe, JL van Haaften. 1975. Mercury and selenium in marine mammals and birds. The
Science of the Total Environment 3:279-287.
8. Levander OA. 1986 Selenium. Pages 209-279 in W. Mertz, ed. Trace Elements in Human and Animal Nutrition. 5th ed. Vol. 2.
Academic Press, Orlando, FL.
9. Lindqvist O. 1994. Atmospheric cycling of mercury: an overview. Pages 181-185 in C.J. Watras and J.W. Huckabee, eds. Mercury
Pollution, Integration and Synthesis. CRC Press Inc., Salem, MA.
10. Magos L. 1997. Physiology and toxicology of mercury. Metal Ions in Biological Systems 34:321-370.
11. Mottet NK, ME Vahter, JS Charleston, LT Friberg. 1997. Metabolism of methylmercury in the brain and its toxicological significance.
Metal Ions in Biological Systems 34:371-403.
12. National Ocean Pollution Program Office. Sept. 1988. Federal plan for ocean pollution research, development and monitoring: fiscal
years 1988-1992. U.S. Department of Commerce, Washington, DC.
13. Petering HG, LB Tepper. 1976. Pharmacology and toxicology of heavy metals: mercury. Journal of Pharmacology and Therapeutics
1:131-151.
14. Puls R. 1988. Mineral levels in animal health, diagnostic data. Sherpa International, British Columbia.
15. Taylor D. 1979. A review of the lethal and sub-lethal effects of mercury on aquatic life. Residue Reviews 72:33-69.
16. Wood JM, FS Kennedy, CG Rosen. 1968. Synthesis of methyl-mercury compounds by extracts of a methanogenic bacterium. Nature
220:173-4.