Richard E. Jones, III; Thomas L. Deardorff, PhD; Stephen C. Kayes, PhD
Ascaridoid nematodes infect a wide variety of terrestrial and aquatic animals. These parasitic nematodes are causitive agents of human diseases such as ascariasis, toxocariasis, and anisakiasis. Although these medically important nematodes are closely related, the diseases they cause and particularly the resulting immunological responses they elicit during infection may vary greatly. We are particularly interested in learning more about the immunological and pathological responses of hosts to the ascaridoid Anisakis simplex.
Anisakis simplex is a marine ascaridoid nematode that is often called lithe herringworm." Humans become exposed to the third-stage larvae of this parasite by consuming infected raw (e.g., sushi, sashimi) or inadequately prepared (e.g., lightly marinated or brined undercooked) seafood products. Once ingested, the larvae may penetrate into or through the gastrointestinal tract. Human anisakiasis is usually characterized by severe epigastric pain, often associated with nausea and vomiting, between one and 12 hours after eating seafood. Peripheral blood eosinophilin may or may not be present in these patients so a detailed history of the patient is often necessary to achieve the differential diagnosis.
Our knowledge of human anisakiasis is primitive compared with what is known about most other ascaridoid diseases. One reason for this paucity of knowledge is the lack of well-defined animal models to study the disease processes. For example, our understanding of human toxocariasis has been greatly facilitated by the demonstration that mice provide excellent models for the human infection and, indeed, develop all the hallmarks of visceral larva migrans (VLM). Perhaps if satisfactory models to study human anisakiasis were established, our knowledge of this disease would increase. Therefore, this paper is concerned with defining the immune responses of mice to the third-stage larvae of A. simplex.
Female CBA/J mice were anesthetized under ether vapor and 2, 5, or 10 viable A. simplex larvae were surgically implanted into the abdominal cavity. The abdominal cavity of control animals were opened but did not receive larvae (sham operations). All surgical incisions were closed with 9 mm wound clips. Mice were sacrificed at 7, 14, or 21 days post-infection.
Spleens weight indices (SWI) of mice sacrificed on days 7, 14, or 21, indicated increasing splenomegaly with increasing worm burden. The SWI was calculated as follows:
Increased spleen weight corresponded with the duration of infection. Variability within each infected group was substantial and we attribute this to different migratory patterns of the worms following implantation into the abdominal cavity. For example, some larvae aggressively penetrate the abdominal viscera whereas others strictly remain embedded in the gut mesentery.
Lymphocyte blast-transformation assays were conducted on single spleen cell suspensions, maintained in 96 well Microtiter plates, utilizing the T-cell mitogen, concanavalin A (Con A), and the B-cell mitogen, lipopolysaccharide (LPS) . After 64 hours of culture, the splenocytes were pulse-labeled with 3H-thymidine (2 mCi/mmole) for eight hours, harvested onto glass filter paper, and the amount of incorporated isotope determined using a scintillation counter. Results of these tests indicated that the nonspecific T-cell response to Con A increased with regard to infection; but not in relation to the size of the worm burden at each period of examination. Representative findings on Day 7 post-infection are presented in Figure 1.

Additionally, Con A mitogenesis increased between days 7 and 14 post-infection but not between days 14 and 21. No differences in LPS-induced B-cell mitogenesis were noted between control and immunized spleen cells in any of the infected groups at any of the three time periods examined.
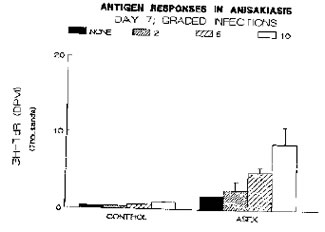
Anisakis simplex excretory-secretory (ASEX) materials with a molecular weight greater than 10 kD were isolated from sterile culture media containing the third-stage larvae and used as a source of antigens in the blast-transformation assay. Like the Con A response, antigen-specific blastogenesis increased in magnitude with increasing worm burden. Naive spleen cells (control groups) also responded to stimulation with ASEX suggesting that some component(s) of ASEX may be mitogenic. Representative data on the antigenic responses for day 7 of these graded infections are presented in Figure 2.
Because patients with chronic anisakiasis often show increased eosinophila, we differentiated the leukocytes in stained peripheral blood smears. Although they did not have increased levels of circulating eosinophils, infected animals displayed an Increased percentage of neutrophils In their peripheral blood and there was not always an accompanying leukocytosis. This finding was true of all durations of infection.
At necropsy, the surgically implanted larvae were located, fixed and sectioned for histopathologic examination. A tendency to become encapsulated in groups in the gut mesentery was characteristic of the larvae from all infection sizes and periods of infection. Lesions associated with these encapsulated groups of larvae were large, well-organized fibrous capsules with inflammatory cells being located between the capsule wall and the worms. Eosinophils were prominent in this inflammatory milieu, even though they were rarely seen in peripheral blood.
The accumulation of eosinophils around the implanted larvae suggests that surface molecules and/or secreted moieties, such as ASEX, may have been released by these helminths into the surrounding tissues and may have specifically attracted eosinophils into these sites. Initial experiments indicate eosinophil chemotactic activity is associated with protein extracts of whole worm homogenate and ASEX when assayed across a 5 um filter in blind-well Boyden chemotaxis chambers.
In conclusion, the surgical implantation of third-stage larvae of A. simplex into the abdominal cavities of CBA/J mice provides a good-but imperfect-model of human anisakiasis. The histologic lesions In both the mouse and the human appear to be quite similar and antibody production against A. simplex by the mouse (data not provided) and human are substantial. Implanted larvae did not induce eosinophilia In the mice (as does larval T. canis in mice and humans); rather, they increased circulating neutrophils. However, eosinophils were present in significant numbers within the capsules surrounding the larvae. Lastly, these results suggest that Anisakis simplex-derived proteins may be chemotactic for eosinophils and mitogenic for B lymphocytes.
Clearly, the implantation of the third-stage larvae of A. simplex into mice can provide insight into the disease processes operative in human anisakiasis as well as the immunobiology of eosinophils as they interact with their parasite targets.
This work was supported in part by a grant from the National Institutes of Health (AI 19968).