The past decade has provided much new information on the pathogenesis of allergic skin disease in animals. This has allowed veterinarians to develop more effective treatment protocols - and safer ways to treat it. Canine atopic dermatitis (AD) has served as the model for allergic skin disease. Much of that science can be applied to other allergies, such as flea allergy dermatitis; cutaneous adverse food reactions (CAFR); and the allergic reactions to parasites, such as Sarcoptes scabiei.
Key Facts
- The cycle of itch/scratch in allergic skin disease is mediated by lymphocytes and the cytokines that they produce.
- Cytokines that utilize Janus kinase (JAK)1 enzymes are primarily involved in the itch and inflammation of allergic skin disease.
- Interleukin (IL)-31 is a key cytokine in many cases of inflammation.
- Traditionally, veterinarians focused on treatment of allergic skin disease with drugs, such as corticosteroids, that targeted controlling pruritus and deceasing inflammation. This was driven by the need to address the owner’s complaints that persistent itching and scratching in their dog was detrimental for their pet and disrupted the quality of life of the pet and the owner.
- Decreasing pruritus remains a key therapeutic objective for veterinarians treating allergic skin disease, and veterinarians understand that treatment typically requires several modes of therapy aimed at both the primary disease as well as the secondary complications.
- Current treatment regimens focus on:
- Identifying and treating infestations, such as fleas, Sarcoptes scabiei, Notoedres cati, Cheyletiella sp., lice, etc.
- Controlling concurrent infections or colonization with common pathogens, such as Staphylococcus pseudintermedius or Malassezia yeast.
- Preventing any allergic conditions that are possible, such as cutaneous adverse food reactions, contact allergies, etc.
- Modifying the immune responses to allergenic stimulation.
- Controlling and breaking the itch/scratch cycle.
- Contemporary, multimodal treatment protocols can include one or more of the following therapeutics based on individual patient needs:
- Topical therapies, such as shampoos, to remove pathogens and allergens and to imp rove the skin barrier
- Moisturizers and restructurant agents to improve the skin barrier
- Nutritional supplements to enhance the skin barrier function
- Antiparasitic products to treat and prevent parasitic infestations
- Anti-infective drugs to treat secondary bacterial and yeast infections
- Hypoallergenic diets
- Antipruritic therapy to control itch and scratching
- Allergen-specific immune therapy (ASIT) to modify the patient’s immune response to specific allergens
- Today, options for treating allergic skin disease, including AD, comprise drugs that are targeted at controlling recently described mechanisms of AD, such as specific cytokines.
Immunopathogenesis of Atopic Dermatitis
In dogs, the immunology of atopic dermatitis starts with a cutaneous Langerhans cell presenting an antigen to a naive T lymphocyte, which it polarizes for the correct response. The immune dysregulation in allergy is a shift toward a T helper (Th) 2 response. The Th2 lymphocytes release cytokines that mediate neuronal itch (such as interleukin [IL]-31) as well as other inflammatory mediators including, IL-2, IL-4, IL-5, IL-6, and IL-13 (Figure 1).
| Figure 1 | 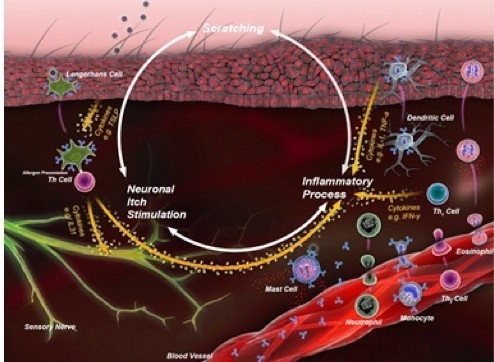
The itch cycle begins with a cutaneous Langerhans cell presenting an antigen to a naive T lymphocyte, which it polarizes for the correct response. The lymphocyte may be “directed” to produce cytokines that classify it as a T-helper (Th) 1 cell. The cytokines primarily kill intracellular pathogens or assist white blood cells to kill extracellular pathogens. In allergy, the immune dysregulation is a shift toward a Th2 response, releasing cytokines that mediate neuronal itch. |
|
| |
Implications for Treatment
Pruritus is the primary reason that owners present their pet with allergic skin disease to the veterinarian. In the past, therapies, such as corticosteroids, were useful in the short term to get a rapid decrease in clinical signs; but the fact that corticosteroids have effects in every cell of the body causes multiple side effects that often outweigh the benefits. Now, based on the new science, there are therapies that are as effective and have fewer side effects.
Given the complexity of the pathogenesis of AD, the goal of treatment is to find the precise combination of therapeutic approaches that effectively provides relief for the individual patient. Multimodal treatment aimed at both the primary disease and at secondary complications can be required. Because many atopic patients require lifelong management, it is also important to consider long-term safety as well as convenience and the cost effectiveness of treatment regimens.
Treatment strategies must address multiple elements:
- Manage itch and control scratching that can perpetuate skin damage.
- Avoid, prevent, or eliminate allergens (such as fleas or foods) when possible.
- Control secondary infections, commonly due to Staphylococcus pseudintermedius or Malassezia yeast, which contribute to discomfort and augment the allergic and inflammatory responses.
- Improve the skin barrier.
- Modify the immunologic response through allergen immunotherapy in patients diagnosed with AD.
Controlling Itch and Inflammation
Fatty Acids (examples: alpha-linolenic acid, eicosapentaenoic acid, docosahexaenoic acid)
Fatty acids are specific kinds of polyunsaturated fats. The two main classes are omega-3 and omega-6, which are based on their molecular characteristics. Oral and topical supplementation with omega-3 fatty acids - alpha linolenic acid (ALA), eicosapentaenoic acid (EPA),or docosahexaenoic acid (DHA) - may help in moderating inflammation and improving the skin barrier (Figure 2). EPA is the big performer of the omega-3 fatty acids and is incorporated into the cell membrane.
| Figure 2 | 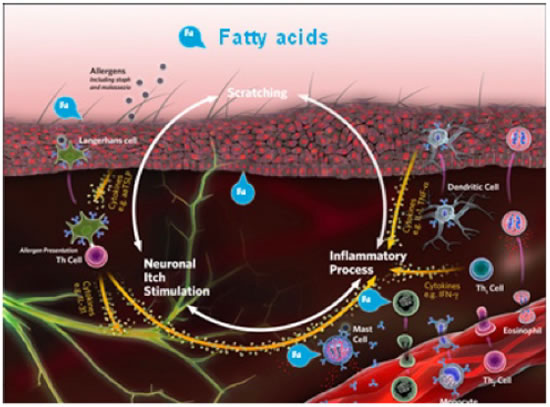
In cases involving itch and inflammation, oral and topical supplementation with omega-3 fatty acids - alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), or docosahexaenoic acid (DHA) - may help in moderating inflammation and improving the skin barrier. |
|
| |
Allergies occur because the pet‘s immune system reacts to normally innocuous proteins, such as house dust and mites. When a cell membrane is damaged, substances, such as arachidonic acid, are released and metabolized by enzymes (lipoxygenase and cyclooxygenase) to proinflammatory byproducts, which add to cutaneous inflammation and pruritus. When omega-3 fatty acids are incorporated into the cell membrane, they utilize the same enzymes and produce less inflammatory substances after cellular damage.
Antihistamines (examples: diphenhydramine, hydroxyzine)
The complexity of the mediators produced by the immune system may explain why there is a poor response to treatment with antihistamines (Figure 3). In fact, antihistamines aimed primarily at H1 and H2 receptors are a common treatment choice in veterinary medicine, but there is little evidence to support their efficacy in the treatment of allergic skin disease. New experimental evidence suggests that targeting H4 receptors may be worthwhile because these receptors are expressed on T lymphocytes, antigen-presenting cells (Langerhans cells), neurons, and keratinocytes.
| Figure 3 | 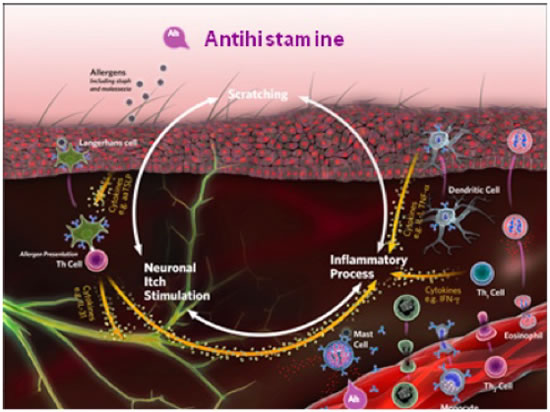
Because of the complexity of the mediators produced by the immune system, treatment with antihistamines typically produces a poor response. Antihistamines aimed primarily at H1 and H2 receptors are a common treatment choice in veterinary medicine, but little evidence supports their efficacy in treating allergic skin disease. |
|
| |
Corticosteroids (examples: prednisolone, dexamethasone, methylprednisolone)
Historically, synthetic corticosteroids are a mainstay in effective treatment of allergic skin disease. Glucocorticoids (GC) are the primary corticosteroids used. As these natural hormones are produced by the adrenal gland and are essential for normal life, they diffuse into and affect the activity of every cell in the body. GC exert most of their actions by binding to intracytoplasmic steroid receptors, which are then transported to the nucleus where they bind to cellular DNA and alter gene expression (Figure 4).
| Figure 4 | 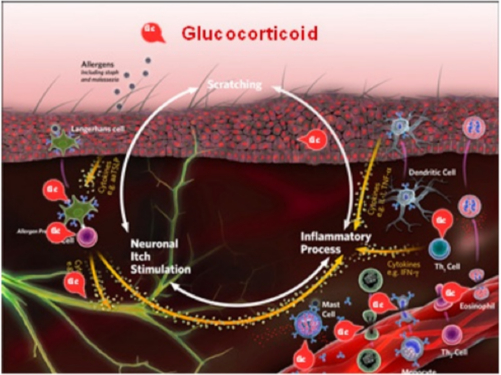
Synthetic corticosteroids, primarily glucocorticoids, are integral in effective treatment of allergic skin disease. GC exert most of their actions by binding to intracytoplasmic steroid receptors, which are then transported to the nucleus where they bind to cellular DNA and alter gene expression. |
|
| |
One of the most important medical functions of corticosteroids is their anti-inflammatory effect. GC stabilize the membranes of lysosomes so that they rupture with difficulty. This helps prevent the usual tissue damage and destruction that occurs when lysosomal enzymes are released. GC also decrease the production of bradykinin, which is a potent vasodilating substance. That decreases the permeability of the capillary membrane, which then prevents protein leakage into inflamed tissues.
GC minimize the inflammatory response through the action of lipomodulin, which inhibits phospholipase A2, the enzyme that normally converts membrane phospholipids into arachidonic acid (AA), a proinflammatory product. The decrease in AA limits available precursor molecules for lipoxygenase and cyclo-oxygenase to produce the AA-derived mediators of inflammation. Lastly, GC inhibit the expression of adhesion molecules on the endothelial cells (particularly ELAM-1 and ICAM-1) and thereby interfere with the movement of leukocytes from the vasculature into inflamed tissues. This is the cause of the commonly noted leukocytosis seen with GC administration.
GC block the inflammatory response to an allergic reaction exactly the same way that they block other types of inflammation. The basic allergic react ion between an antigen and antibody is not affected, and even some of the secondary effects of the allergic reaction, such as the release of histamine, still occur. However, the subsequent inflammatory response is responsible for many of the serious and sometimes fatal effects of the allergic reaction. Administration of GC can be lifesaving.
The specific GC effects that occur with their therapeutic use are summarized here and should be considered, particularly if ongoing therapy will be needed:
- Suppress the release of AC TH by the pituitary gland and, therefore, suppress the release of corticosteroids by the adrenal cortex.
- Reduce the number of circulating lymphocytes through redistribution and suppress T lymphocyte function
- Reduce the number of circulating eosinophils
- Help maintain cell membrane activity
- Inhibit macrophage function
- Suppress antibody production
- Inhibit the release of endogenous pyrogen (IL-1)
- Depress prostaglandin and leukotriene synthesis
- Alter the complement and kinin cascades
- Interfere with leukocyte migration and adhesion
- Suppress lysosomal release from neutrophils by stabilizing lysosomal membranes
- Suppress phagocytosis
- Reduce fibroblast activity resulting in delayed healing and thinning of the skin
- Affect enzyme actions and other cellular functions
Calcineurin Inhibitors (examples: ciclosporin, tacrolimus)
Ciclosporin (Atopica® - Novartis), is a calcineurin inhibitor that suppresses the production of inflammatory cytokines by T lymphocytes (Figure 5). Because these cytokines are upstream of the actual itch event and because they are not the only mediators of disease and pruritus, these medications can take several weeks to be fully effective. Studies have shown that the signs of canine AD can be well controlled with the use of ciclosporin, 5 mg/kg every 24 hours, in approximately 50% to 70% of cases, with increasing percentages during longer-term use. Major advantages include the lack of steroid-related side effects, such as polyuria, polydipsia, weight gain, muscle weakness, and personality changes. The major disadvantage to the use of this drug is the cost, but that can be lessened with every-other-day or half-dose-daily therapy, which is attainable in 40% to 50% of cases.
| Figure 5 | 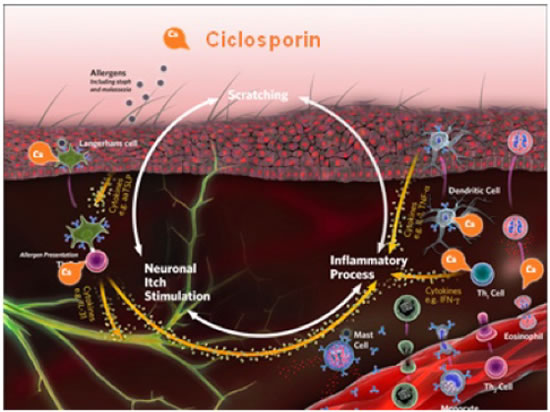
Ciclosporin is a calcineurin inhibitor that suppresses the production of inflammatory cytokines by T lymphocytes. Because the cytokines are released above the actual itch event and because they are not the only mediators of disease and pruritus, medications can take several weeks to be fully effective. |
|
| |
Gastrointestinal side effects occur in approximately 15% to 40% of cases but are usually self-limiting and can be minimized by slowly building up to the full dose over 10 to 14 days, pre-treating with an antiemetic 2 hours before cyclosporin for the first 10 days, freezing the capsules, or giving medication with food. Other less common side effects include papillomatosis, hirsutism, gingival hyperplasia, tremors/neuropathies, secondary pyoderma, and lymphoplasmacytic dermatitis. Occasional hypoalbuminemia, urinary tract infections, and increased liver enzymes may be seen.
Janus Kinase Inhibitors (example: oclacitinib)
Oclacitinib (Apoquel® - Zoetis) is a Janus kinase (JAK) inhibitor that is labeled for the control of pruritus associated with allergic dermatitis and control of atopic dermatitis in dogs at least 12 months of age. The mode of action is to bind to and block the activity of the JAK enzyme attached to the cellular cytokine receptors that utilize the JAK1 enzyme. Most of the cytokines involved in allergic skin disease utilize these types of receptors (Figure 6). Oclacitinib has been shown to control itch and inflammation as well as oral prednisolone does in client-owned dogs with allergic skin disease due to fleas, food, contactants, or atopic dermatitis without many of the side effects associated with the administration of corticosteroids. Dogs administered prednisolone had an increase in adverse effects, most importantly an increase in liver enzymes. In laboratory studies, oclacitinib is more rapid-acting (within 1 hour) than oral prednisolone or dexamethasone injections in laboratory beagles administered IL-31.
| Figure 6 | 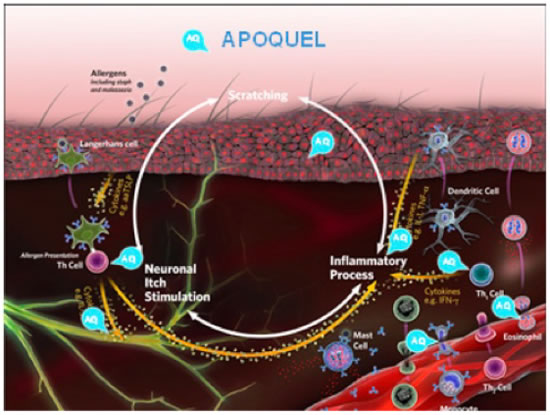
Oclacitinib is a Janus-kinase (JAK) inhibitor that is labeled for control of pruritus associated with allergic dermatitis and control of atopic dermatitis in dogs at least 12 months of age. It binds to and blocks the activity of the JAK enzyme attached to the cellular cytokine receptors that utilize the enzyme. |
|
| |
Monoclonal Antibodies (example: Cytopoint®)
With new information on the role of interleukin-31 (IL-31) in dogs with atopic dermatitis, a caninized anti-cIL-31 monoclonal antibody has been developed (Cytopoint®, Zoetis). Whereas Apoquel® works by binding and blocking the JAK enzyme used by a wide variety of cytokines, working after the cytokines bind the receptor, Cytopoint® neutralizes only the IL-31 cytokine before binding to the receptor (Figure 7). When a single injection of 2 mg/kg was administered to client-owned dogs with atopic dermatitis, 82% of dogs experienced clinical success in reduction of pruritus (2 cm reduction on a 10 cm scale) by day 3. Thirty-eight percent achieved 50% or greater reduction in dermatitis scores (as measured by the CADESI-03 severity scale) 14 days after administration of the antibody and 52% by day 42. Side effects were approximately equal between dogs administered the antibody or placebo.
| Figure 7 | 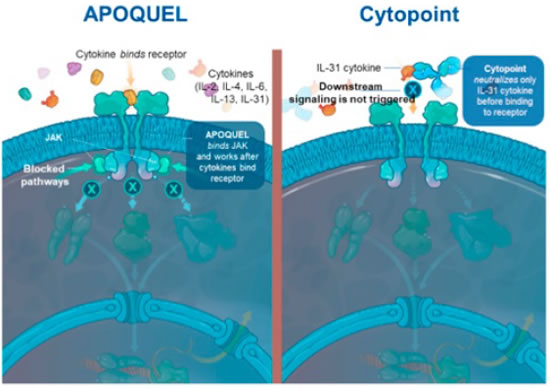
Recent information on the role of interleukin-31 (IL-31) in dogs with atopic dermatitis, led to development of a caninized anti-cIL-31 monoclonal antibody (Cytopoint®). Whereas Apoquel® works by binding and blocking the JAK after the cytokines bind the receptor, Cytopoint® neutralizes only the IL-31 cytokine before binding to the receptor. |
|
| |
Allergy Testing and Immunotherapy
Allergen-specific immunotherapy (ASIT) is a treatment for AD in dogs and cats wherein extracts of allergens to which the patient is sensitive are injected or administered orally, in gradually increasing amounts, to lessen or reverse the hypersensitivity state (Figure 8). Allergy testing, either intradermal or allergen-specific IgE serology, is used as a prelude to ASIT. Allergy tests do not answer the question “Is this pruritic patient atopic?” because some normal and nonatopic patients also have positive test results. In addition, a small percentage of dogs diagnosed with atopic dermatitis have no measurable increase in allergen-specific IgE. This is referred to as “intrinsic” AD.
| Figure 8 | 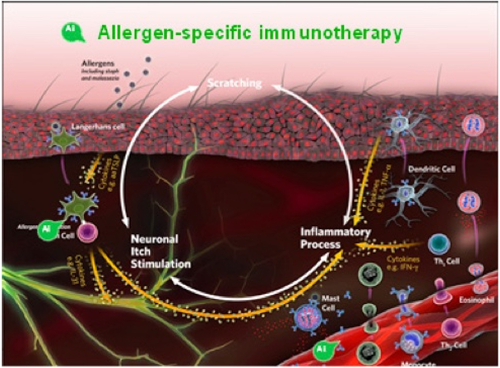
Allergen-specific immunotherapy (ASIT) is a treatment for AO in dogs and cats wherein extracts of allergens to which the patient is sensitive are injected or administered orally, in gradually increasing amounts, to lessen or reverse the hypersensitivity state. Intradermal or allergen-specific IgE allergy testing precedes ASIT. |
|
| |
Allergy tests are useful in those patients where a diagnosis of AD has been made by ruling out other causes of pruritus and, therefore, are candidates for ASIT. This is the only treatment that may reverse part of the underlying pathogenesis of the disease. Immunotherapy is thought to normalize the immune response by increased production of T regulatory cells and anti-inflammatory cytokines that reduce the Th2 inflammatory cascade. The chief disadvantages are that it takes several months or more to begin working and that only 60% to 70% of dogs experience a “good-to-excellent” response (defined as at least 50% improvement in clinical signs) after 1 year of treatment.
References
1. Cosgrove SB, Wren JA, Cleaver DM, et al. A blinded, randomized, placebo-controlled trial of the efficacy and safety of the Janus kinase inhibitor oclacitinib (Apoquel) in client-owned dogs with atopic dermatitis. Vet Dermatol. 2013;24:58–7e142.
2. Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479–e114.
3. Cowden JM, Zhang M, Dunford PJ, Thurmond RL. The histamine H4 receptor mediates inflammation and pruritus in Th2-dependent dermal inflammation. J Invest Dermatol. 2010;130:1023–1033.
4. Ezzat MH, Hasan ZE, Shaheen KY. Serum measurement of interleukin-31 (IL-31) in paediatric atopic dermatitis: elevated levels correlate with severity scoring. J Eur Acad Dermatol Venereol. 2011;25:334–339.
5. Flohr C, Yeo L. Atopic dermatitis and the hygiene hypothesis revisited. Curr Probl Dermatol. 2011;41:1–34.
6. Gadeyne C, Little P, King VL, Edwards N, Davis K, Stegemann MR. Efficacy of oclacitinib (Apoquel®) compared with prednisolone for the control of pruritus and clinical signs associated with allergic dermatitis in client-owned dogs in Australia. Vet Dermatol. 2014;25:512–e86.
7. Grimstad O, Sawanobori Y, Vestergaard C, et al. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Exp Dermatol. 2009;18:35–43.
8. Linek M, Favrot C. Impact of canine atopic dermatitis on the health-related quality of life of affected dogs and quality of life of their owners. Vet Dermatol. 2010;21:456–462.
9. Little PR, King VL, Davis KR, Cosgrove SB, Stegemann MR. A blinded, randomized clinical trial comparing the efficacy and safety of oclacitinib and ciclosporin for the control of atopic dermatitis in client-owned dogs. Vet Dermaotol. 2015;26:23–eB.
10. Marsella R, Olivry T, Carlotti DN for the International Task Force on Canine Atopic Dermatitis. Current evidence of skin barrier dysfunction in human and canine atopic dermatitis. Vet Dermatol. 2011;22:239–248.
11. Metz M, Grundmann S, Stander S. Prutitus: an overview of current concepts. Vet Dermatol. 2011;22:121–131.
12. Michels GM, Ramsey OS, Walsh KF, et al. A blinded, randomized, placebo-controlled, dose determination trial of lokivetmab (ZTS-00103289), a caninized, anti-canine IL-31 monoclonal antibody in client-owned dogs with atopic dermatitis. Vet Dermatol. 2016;27:478–e129.
13. Michels GM, Walsh KF, Kryda KA, et al. A blinded, randomized, placebo-controlled trial of the safety of lokivetmab (ZTS-00103289), a caninized anti-canine IL-31 monoclonal antibody in client-owned dogs with atopic dermatitis. Vet Dermatol. 2016;27:505–e136.
14. Mommert S, Gschwandtner M, Gutzmer R, Werfel T. The role of the histamine H4 receptor in atopic dermatitis. Curr Allergy Asthma Rep. 2011;11:21–28.
15. Olivry T, Mueller RS for the International Task Force on Canine Atopic Dermatitis. Evidence-based veterinary dermatology: a systematic review of the pharmacotherapy of canine atopic dermatitis. Vet Dermatol. 2003;14:121–146.
16. Olivry T, DeBoer D, Favrot C, et al. Treatment of canine atopic dermatitis: 2010 clinical practice guidelines from the International Task Force on Canine Atopic Dermatitis. Vet Dermatol. 2010;21:233–248.
17. Olivry T, Bizikova P. A systematic review of randomized controlled trials for prevention or treatment of atopic dermatitis in dogs: 2008–2011 update. Vet Dermatol. 2013;24:97–2e6.
18. Olivry T, DeBoer D, Favrot C, et al. Treatment of canine atopic dermatitis: 2015 updated guidelines from the International Committee on Allergic Diseases of Animals (ICADA). BMC Vet Res. 2015;11:210.
19. Raap U, Standers, Metz M. Pathophysicology of itch and new treatments. Curr Opin Allergy Clin Immunol. 2011;11:420–427.
20. Rahman S, Collins M, Williams CMM, Ma HL. The pathology and immunology of atopic dermatitis. Inflamm Allergy Drug Targets. 2011;10:486–496.