Stanley L. Marks, BVSc, PhD, DACVIM (Internal Medicine, Oncology), DACVN
Dysphagia is defined as an abnormality in swallowing, and is frequently a diagnostic challenge for veterinarians. The causes of dysphagia may be secondary to either a neurologic or muscular disturbance of the swallowing reflex (functional) or secondary to strictures, traumatic injury, foreign bodies, or neoplastic processes (structural) involving the oropharyngeal region or esophagus. The swallowing reflex involves a coordinated process involving the tongue, hard and soft palate, pharyngeal muscles, esophagus, and gastroesophageal junction. The swallowing reflex is also dependent on normally functioning striated muscle and neuromuscular transmission, the integrity of the trigeminal, facial, glossopharyngeal, vagus, and hypoglossal nerves, their nuclei in the brainstem, and the swallowing center in the reticular formation of the brain.
Dysphagia is a relatively common sign in dogs and is less commonly seen in cats. The animal's signalment is important to consider as dysphagic puppies and kittens and younger dogs or cats can be diagnosed with a variety of congenital disorders causing abnormal swallowing. In addition, caution must be heeded in differentiating clinical signs of dysphagia, regurgitation, vomiting, retching, and gagging. Failure to differentiate these signs during the clinical examination can result in inappropriate diagnostic testing and a likely misdiagnosis.
Key Point: It is important to remember that dysphagia is not a specific disease, but a clinical sign caused by a variety of functional and structural disorders affecting the swallowing reflex.
Anatomical Localization of Dysphagia
Dysphagia may be further classified based on cineradiographic analysis according to the anatomic area in which the swallowing dysfunction originates. Patients affected by oropharyngeal dysphagia make exaggerated swallowing movements and food will usually drop from the mouth within seconds of prehension. In contrast, esophageal dysphagia results in more delayed regurgitation and is usually not associated with exaggerated swallowing movements. Gastroesophageal dysphagias are well documented in people, and are being increasingly recognized in dogs and cats secondary to hiatal hernias and decreased tone in the lower esophageal sphincter. It is pivotal to determine whether dysphagia is a sole presenting clinical sign or is associated with multiple clinical abnormalities.
Oropharyngeal dysphagia can be divided into 3 stages based on the site of dysfunction; the oral, pharyngeal, and cricopharyngeal stages. In oral dysphagia, the animal has difficulty prehending and transporting food or water to the oropharynx. The pharyngeal stage requires the active propulsion of the food bolus by the tongue to the caudal pharynx, followed by pharyngeal constriction of the bolus towards the upper esophageal sphincter. The cricopharyngeal stage involves the relaxation of the cricopharyngeal muscles (upper esophageal sphincter) and passage of the bolus into the proximal esophagus. Normal passage of the bolus into the esophagus is dependent on synchrony between the constriction of the pharyngeal muscles and relaxation of the cricopharyngeus muscle. Failure of the bolus to pass through the cricopharyngeus region is called cricopharyngeal dysphagia. Table 1 contains a list of differential diagnoses for disorders affecting each of the anatomical regions affecting the swallowing reflex.
Table 1.
|
Anatomic localization |
Differential diagnoses |
|
Oropharyngeal dysphagias |
|
|
Oral phase |
Cranial nerve V, VII and XIII dysfunction, skull fractures, oral masses or foreign bodies |
|
Pharyngeal phase |
Pharyngeal abscess, foreign body or mass, Cranial nerve V, VII, X, and IX dysfunction resulting in pharyngeal paresis/paralysis |
|
Cricopharyngeal phase |
Cricopharyngeal achalasia, cricopharyngeal dyssynchrony |
|
Esophageal dysphagias |
Primary (idiopathic) megaesophagus, secondary megaesophagus, esophageal stricture, mass, granuloma, foreign body, extra-esophageal compression, esophageal diverticulum |
|
Gastroesophageal dysphagias |
Hiatal hernia, periesophageal hernia, gastroesophageal intussusceptions, reflux esophagitis |
Diagnosis of Swallowing Disorders
1. History: The age of onset of the dysphagia and the breed should be determined. Congenital problems are usually due to idiopathic megaesophagus or vascular ring anomalies, but occasionally foreign bodies or cricopharyngeal dysphagia will be diagnosed. If the patient shows signs of dysphagia in adulthood, the likelihood of a recent anesthesia or exposure to caustic chemicals, foreign bodies, or toxic agents should be evaluated. Gastroesophageal reflux is a relatively common phenomenon in anesthetized patients, and can result in secondary esophagitis with potential stricture formation. Reports of concurrent muscle weakness or neurologic disorders should alert the clinician to an extraesophageal disease process that may underlie the esophageal disease. Information regarding the animal's ability to retain liquids, soft food, or solid food should be obtained. It is essential to obtain an accurate description of the dysphagia.
2. Physical Exam: This must include careful examination of the pharynx (using sedation or anesthesia if necessary). The pharynx and neck should be carefully palpated for masses, asymmetry, or pain. The chest should be carefully auscultated for evidence of inhalation pneumonia. Evaluation of cranial nerves should be performed including assessment of tongue and jaw tone, and abduction of the arytenoid cartilages with inspiration. Complete physical and neurological examination may identify clinical signs supportive of a generalized neuromuscular disorder, including muscle atrophy, stiffness, or decreased/absent spinal reflexes.
Key Point: One of the most valuable diagnostic tests in dysphagic animals is to observe the animal ingesting whatever food (or liquid) the animal has difficulty swallowing. Close observation will usually separate dysphagia into oral, pharyngeal, cricopharyngeal, or esophageal causes.
3. Cervical and Thoracic Radiographs: The pharynx of normal animals is evident on radiographs because it is air filled. The size of the air-filled space can be decreased by local inflammation or neoplasia, laryngeal edema, or elongation of the soft palate. Pharyngeal size can also appear increased with dysfunction of the pharynx or upper esophageal sphincter, chronic respiratory (inspiratory) disease, and chronic severe megaesophagus. The normal esophagus is not visible on survey radiographs. An exception occurs following aerophagia due to excitement, nausea, dyspnea, or anesthesia.
4. Barium Contrast Videofluoroscopy: Videofluoroscopy can define normal pharyngeal and upper esophageal anatomy (Figure 1) and is used to evaluate and classify functional oropharyngeal dysphagias. The animal is fasted for 12 hours prior to the procedure and survey radiographs of the thorax and cervical region are obtained to rule out gross abnormalities. The patient is then placed in lateral or sternal recumbency on the fluoroscopy table. Liquid barium sulfate suspension is administered orally in small (5-15 ml) boluses and at least 3 swallows should be observed. At least 1 bolus should be followed all the way to the stomach. Recording the study and performing frame-by-frame analysis can provide quantitative measures of swallowing function in subtle or debatable cases of asynchronous UES opening. The normal timing of the swallowing act has been reported. A UES opening time value greater than 2 standard deviations from the normal mean is considered delayed/dyssynchronous. Barium soaked kibble should also be given orally and bolus propulsion observed fluoroscopically so as to rule out a functional abnormality that allows liquid passage but affects swallowing of solid foods.
Key Point: A dynamic study utilizing videofluoroscopy is essential to diagnose disorders affecting the cricopharyngeal stage of swallowing.
Click on the image to see a larger view.
| Figure 1. | 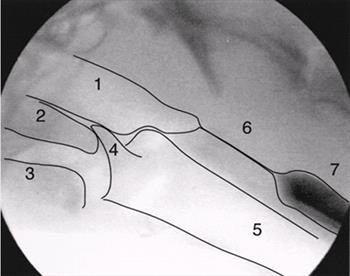
A single fluoroscopic frame of a normal dog is shown to define the normal pharyngeal anatomy.
1=nasopharynx, 2=soft palate, 3=base of tongue, 4=epiglottis, 5=trachea, 6=upper esophageal sphincter, 7=cranial esophagus. |
|
| |
5. Endoscopy (esophagoscopy) with either a rigid or flexible fiberoptic scope is an important tool for evaluating esophageal disease. Foreign bodies can be visualized and retrieved, strictures can be ballooned, and esophageal biopsies can also be procured for histologic confirmation of suspected esophagitis.
6. Esophageal manometry is a useful technique to evaluate esophageal pressure and motor function. It is utilized extensively in human medicine and on research animals, but has not been widely applied in clinical veterinary medicine.
7. Electromyography (EMG) can be helpful in localizing the dysphagia and evaluating myopathic causes of dysphagia.
8. Muscle biopsy is an invaluable diagnostic tool for confirming myopathic causes of dysphagia.
Specific Diseases of the Oropharynx and Esophagus
1. Cricopharyngeal Dysphagia
The normal swallowing reflex is initiated when food or fluid is passed to the base of the tongue. A peristaltic wave of contraction beginning in the cranial pharynx moves the food toward the esophagus. The epiglottis closes to prevent aspiration of food and the upper esophageal sphincter composed of the cricopharyngeus and thyropharyngeus muscles relaxes and allows food to pass into the esophagus. Peristaltic contractions then pass the food in an aboral direction toward the stomach.
Key Point: Failure of the upper esophageal sphincter to relax or incoordination between UES relaxation and pharyngeal contraction results in an inability to pass food or fluids into the esophagus and is termed cricopharyngeal dysphagia.
In dogs with cricopharyngeal dysphagia, food or fluid accumulates in the pharynx, followed by gagging and retching. The reflex integration of swallowing and respiration (i.e., closure of the epiglottis) may also be abnormal, predisposing the animal to aspiration pneumonia.
The etiology of cricopharyngeal dysphagia is unknown. An acquired abnormality anywhere in the reflex arc (afferent or efferent nerves, brain stem, neuromuscular junction, muscle) could cause this disorder. The cause in a few dogs is fibrosis of the cricopharyngeus muscle which can be determined upon pharyngeal examination and fluoroscopic evaluation. Cricopharyngeal dysphagia can also be a manifestation of generalized neuromuscular diseases such as polyneuropathies, polymyositis, and myasthenia gravis. The disease has been reported as a congenital lesion in cocker and springer spaniels and is often associated with toy breeds. Research at UC Davis has also identified a relatively large population of Golden Retrievers with this abnormality, although the mode of inheritance has not been elucidated.
Animals with cricopharyngeal dysphagia usually present with a history of regurgitation of solid food immediately after eating, either through the nose or mouth. Some dogs can present with regurgitation of water in association with cricopharyngeal dysphagia. Repeated swallowing attempts, coughing, spluttering, and sneezing are all characteristic. If the problem is of long standing duration affected animals may be emaciated but are generally bright and alert with a good appetite.
Diagnosis of cricopharyngeal dysphagia requires a complete examination of the pharynx for other causes (pharyngitis, foreign body, abscess, neoplasia). Plain radiographs are non-diagnostic so positive contrast radiography using fluoroscopy is necessary to confirm the diagnosis. Fluoroscopy reveals a failure of the upper esophageal sphincter to relax normally and reflux of contrast material. Thoracic radiographs should also be obtained to evaluate for the presence of aspiration pneumonia or concurrent megaesophagus. Electromyography of the muscles associated with swallowing as well as the tongue and proximal esophagus can also be helpful in localizing the lesion and identifying the cause of dysphagia.
Treatment of abnormal sphincter function has involved surgical myotomy or myectomy of the cricopharyngeus muscle, although this procedure is controversial. The prognosis for uncomplicated sphincter abnormalities after surgical myotomy is guarded, as some dogs can have exacerbation of their dysphagia following surgery. If pharyngeal motility abnormalities are present, the prognosis is poor. In a series of 14 dogs recently diagnosed with cricopharyngeal dysphagia and managed via myotomy or myectomy of the cricopharyngeus muscle, 6 dogs had no improvement following surgery, 5 dogs had partial improvement in their swallowing, and 3 dogs had complete resolution of their dysphagia. Conservative management of motility problems involves altering the consistency of the food to determine what type is the easiest for the animal to swallow. The biggest post-surgical complication of cricopharyngeal myotomy or myectomy is a predisposition to aspiration pneumonia. Many patients unable to swallow are managed with esophagostomy tubes or gastrostomy tubes to decrease the incidence of aspiration pneumonia and help ensure that the patient's caloric requirements are met.
2. Megaesophagus
Generalized loss of motor function to the esophagus results in dilatation and loss of normal peristaltic motility. As a result, food and fluid accumulate in the esophagus.
Idiopathic megaesophagus is the most common type of megaesophagus in the dog and cat. The syndrome may be manifested either in puppies at the time of weaning or in adulthood. The etiology of idiopathic megaesophagus is unknown. The congenital form of the disease may be due to a delay in maturation of the esophageal neuromuscular system; a theory that explains why young dogs may improve with careful feeding management. Idiopathic megaesophagus has been shown to be inherited in the wire-haired fox terrier and the miniature schnauzer. A breed predisposition also exists for the German Shepherd, Great Dane and Irish Setter. The site and pathogenesis of the lesion in idiopathic megaesophagus is unknown. Suggested hypotheses include abnormalities of the afferent limb of the reflex arc (receptors, neurons) or of the swallowing center in the CNS. Idiopathic megaesophagus may also occur rarely in the cat.
Secondary megaesophagus may result from a large number of systemic diseases including, myasthenia gravis, SLE, polymyositis, polymyopathies, dermatomyositis, polyneuropathies, dysautonomia, botulism, distemper, neoplasia, brain stem disease, lead and thallium toxicity, Addison's disease, hypothyroidism, pituitary dwarfism, and thymoma. Many obstructive esophageal diseases (neoplasia, granuloma, vascular ring anomaly, stricture, periesophageal masses and foreign bodies) can also lead to megaesophagus if they are of sufficiently chronic duration.
Dogs with megaesophagus usually present for regurgitation which often occurs immediately or several hours after eating. The food is undigested and usually has an alkaline pH. Dysphagia is not generally present. Anorexia, drooling, and pain on swallowing may be seen secondary to esophagitis which can develop due to the accumulation of food in the esophagus. Animals with megaesophagus are predisposed to aspiration pneumonia.
Key Point: Reliance on the timing of the regurgitation event in relation to the ingestion of a meal must be interpreted with caution, as many dogs can regurgitate many hours following a meal.
Diagnosis: Megaesophagus is diagnosed based on the history, clinical signs, and thoracic radiography. Survey thoracic radiographs may be diagnostic if the cervical esophagus is dilated by air or if the thoracic esophagus contains large amounts of food or fluid. Dogs that are excited or animals that are anesthetized frequently have air in the esophagus which is a normal phenomenon. Positive contrast studies utilizing fluoroscopy may be required to confirm esophageal dysfunction and to evaluate esophageal motility. Enlargement of the proximal esophagus with normal function of the distal esophagus suggests a vascular ring anomaly or esophageal stricture. Once a diagnosis of generalized megaesophagus is confirmed, diagnostic testing should be performed to establish an underlying etiology if possible.
Diagnostic tests that should be considered to evaluate animals with generalized megaesophagus include a complete blood count, biochemical panel, urinalysis, blood lead level, creatine kinase concentration, acetylcholine receptor antibody test (for evaluation of myasthenia gravis), and evaluation of adrenal and thyroid gland function. Additional diagnostic procedures that can be performed based on the animal's signalment, history, and neurological examination include an EMG, nerve conduction velocities, and muscle biopsies. Videofluoroscopy is essential for the diagnosis of functional esophageal disorders (esophageal dysmotility) not associated with esophageal dilation and has some prognostic value in megaesophagus via assessment of the severity of peristaltic dysfunction. Esophagoscopy is less reliable than radiography and fluoroscopy, although it can be used to rule out underlying causes of megaesophagus such as esophagitis, neoplasia, and radiolucent foreign bodies.
Medical management of generalized megaesophagus involves modification of feeding practices. Dogs with megaesophagus generally tolerate a liquid or semi-liquid gruel better than solid food. Feeding from an elevated position allows gravity to help move the liquid into the stomach. If possible the animal should be held in a vertical position for 5-10 minutes after eating. Multiple feedings rather than one large single meal may also help minimize food accumulation in the esophagus. We have successfully placed low-profile-gastrostomy tubes for feeding in many dogs with idiopathic megaesophagus in an effort to minimize aspiration pneumonia. The silicon tubes used are extremely durable and are usually replaced on a yearly basis. The frequency of aspiration pneumonia has been markedly reduced in comparison to oral feeding and this therapeutic modality should be considered when a client is willing to dedicate the time to tube maintenance and feeding. The prognosis for dogs with megaesophagus is very variable depending upon the underlying etiology, the degree of dysfunction and the systemic status of the dog. The long term prognosis is poor in most cases, although some cases can be managed successfully for years. The prognosis is improved if treatment of an underlying disease is possible.
3. Esophageal Obstruction
Esophageal obstruction may be intraluminal, intramural, or periesophageal. Most intraluminal obstructions are caused by foreign bodies, most commonly bones and fish hooks. Objects tend to become caught in the thoracic inlet, at the base of the heart and at the diaphragmatic hiatus, which are the areas of least distensibility. Since foreign bodies stimulate peristalsis, severe ulcerative esophagitis, esophageal perforation (causing pleuritis or mediastinitis) or stricture formation can be the result.
The clinical signs of an esophageal foreign body include anorexia, adipsia, depression, hypersalivation, and drooling. Occasionally regurgitation is observed. A definitive diagnosis is made by radiography. Most commonly survey radiographs are adequate, however radiolucent objects may require barium swallow for visualization. Barium should not be given if esophageal perforation is suspected, because this can cause granulomas if it leaks into the mediastinum. In this case an iodinated compound should be administered, or endoscopy should be utilized to visualize the foreign body. If available, endoscopic diagnosis is preferable to barium contrast studies, even if perforation is not suspected, since diagnosis and removal can often be accomplished in one step.
Treatment involves removal of the foreign body by either endoscopy or surgery. Endoscopy is the technique of choice unless the object is deeply embedded, or has perforated through the esophageal wall. Complications associated with esophageal foreign bodies include mediastinitis secondary to perforation, aspiration pneumonia, stricture formation or segmental hypomotility.
Intramural esophageal obstruction is usually a result of stricture formation. Strictures develop when esophageal erosions extend into the muscle layer of the esophagus stimulating the production of fibrous tissue. Esophageal foreign bodies and reflux esophagitis which may occur during abdominal surgery are the usual causes of stricture formation. The primary clinical sign associated with esophageal stricture is regurgitation. Diagnosis relies on positive contrast radiography and endoscopic visualization. Esophageal dilatation may occur cranial to the stricture and abnormal esophageal motility may also occur.
Treatment of esophageal stricture(s) can be challenging. Medical treatment involves decreasing esophagitis by feeding the animal through a gastrostomy tube and dilatation of the stricture using balloon catheter dilators (passed through an endoscope under direct vision, or under fluoroscopic guidance). Esophageal strictures may require repeated dilation at 3 to 4 day intervals for up to 6 procedures to prevent restricturing. Some authors recommend administration of oral or intralesional corticosteroids given at anti-inflammatory doses after dilation to inhibit the formation of new fibrous connective tissue. While total resolution of strictures is not usually possible, clinical signs can usually be kept to a minimum by dietary manipulations such as feeding a liquefied diet. Sucralfate (Carafate) is a basic aluminum salt of a sulfated disaccharide that binds selectively to proteins at the ulcer site by way of electrostatic interactions. Sucralfate is an excellent drug to help manage patients with strictures associated with esophagitis. An H2- receptor antagonist such as famotidine or a more potent antacid such as omeprazole are frequently administered to increase the intragastric pH, and minimize esophageal mucosa damage from refluxed gastric juice. Surgical resection should be reserved for those cases in which no response is seen to medical treatment, or when the lesion is very near to the gastroesophageal junction.
Peri-esophageal causes of esophageal obstruction include inflammation, neoplasia, severe cardiomegaly, hilar lymphadenopathy, and vascular ring anomalies. The most common vascular ring anomaly is persistent right aortic arch (PRAA). In this anomaly the aortic arch develops from the right rather than the left fourth aortic arch. As a result the esophagus becomes constricted by a ring formed by the aorta on the right, the pulmonary artery on the left, the ligamentum arteriosum dorsally and the trachea ventrally. Irish Setters, German Shepherd dogs, and Boston Terriers are the most commonly affected by PRAA. PRAA has also been reported rarely in the cat. Food is unable to pass through the vascular ring and accumulates in the cranial esophagus, causing dilatation and loss of esophageal motility. Clinical signs of regurgitation and poor weight gain are first observed when puppies are weaned onto solid food. Diagnosis is by positive contrast radiography which usually reveals esophageal dilation cranial to the base of the heart and a normal esophagus caudal to the heart. Therapy involves surgical resection of the ligamentum arteriosum, however irreversible esophageal damage may cause some clinical signs to persist in 67% of cases. No improvement is seen in 24% of cases.
4. Esophagitis
Inflammation of the esophagus may result from the ingestion of caustic agents, chronic vomiting, foreign body obstruction, or reflux esophagitis. Reflux esophagitis results from loss of competency of the gastroesophageal sphincter (lower esophageal sphincter) and subsequent reflux of gastric contents and acid into the distal esophagus. The etiology is not known although translocation of the abdominal section of the esophagus (which is responsible for maintaining a competent gastroesophageal sphincter) into the thorax has been suggested as a cause. This can occur due to neuromuscular disease, neoplasia, hiatal hernias, abdominal surgery, pharyngostomy tube placement, or protracted vomiting. Reduced gastroesophageal sphincter tone has also been reported in dogs with megaesophagus, with inspiratory dyspnea, and following the administration of a number of drugs. Lastly, animals that are under general anesthesia are at increased risk for gastroesophageal reflux, as many of the induction agents decrease the lower esophageal sphincter pressure, and the position of the animal increases the likelihood of reflux.
Clinical signs of esophagitis are dependent on the severity of the inflammation. With mild inflammation no clinical signs or just intermittent regurgitation may be evident. With severe inflammation, anorexia, adipsia, hypersalivation, depression, regurgitation, odynophagia (painful swallowing), and weight loss may be seen.
Diagnosis: Positive contrast radiography will often reveal random and uncoordinated esophageal contractions. Barium tends to move easily in both the oral and aboral directions and is frequently retained in the inflamed portions of the esophagus. Occasional gastroesophageal reflux in normal dogs is not considered abnormal if the refluxed ingesta is rapidly returned to the stomach. Definitive diagnosis of esophagitis requires endoscopy and esophageal biopsy which can be obtained with endoscopy biopsy forceps. Gross mucosal findings include mucosal erythema, edema, ulceration and hemorrhage.
Therapy for esophagitis involves removing the underlying cause if possible, discontinuation of oral feeding for 24-48 hours (for severe esophagitis), followed by small frequent feedings of a fat-restricted diet and neutralization or inhibition of gastric acid secretion. Oral antacids, such as Amphojel, Basaljel, or Di-Gel are not recommended as they need to be given up to six times a day to prevent rebound gastric acid hypersecretion. H2-receptor antagonists such as ranitidine or famotidine are particularly effective in reducing parietal cell acid secretion and usually more convenient for owners to administer. Prokinetic agents such as cisapride can be used to increase lower esophageal sphincter pressure and increase gastric emptying. Cisapride will decrease gastroesophageal reflux by tightening the lower esophageal sphincter.
Key Point: Cisapride should not be used in dogs with megaesophagus as the drug only works on smooth muscle. Because the entire length of the canine esophagus is composed of striated muscle, cisapride would have no effect on the esophagus in a dog with megaesophagus, but would tighten the lower esophageal sphincter (which contains smooth muscle fibers), which could have disastrous consequences.
The diffusion-barrier drug, sucralfate is also valuable in managing patients with severe esophagitis as the drug will coat ulcerated mucosa and act as a barrier. Omeprazole, a proton-pump inhibitor, is a potent antacid, and is reserved for patients with severe reflux esophagitis. A combination of cisapride, sucralfate, and omeprazole is very effective in the management of severe esophagitis caused by persistent vomiting, gastroesophageal reflux, foreign body induced trauma and surgical manipulations.
5. Esophageal Neoplasia
Neoplasia of the esophagus is very rare in small animals. Leiomyomas, carcinomas, and sarcomas have been reported. In the southeastern portions of the United States, Spirocerca lupi infections can induce fibrosarcomas and osteosarcomas in the esophagus.
Specific Diseases of the Gastroesophageal Region
Gastroesophageal dysphagias may present with clinical signs of regurgitation, hematemesis, or respiratory distress. Cranial abdominal pain may also be found. Barium contrast videofluoroscopic esophagography can help diagnose and differentiate between many of these disorders.
Key Point: Transient lesions such as hiatal hernias and gastroesophageal reflux may manifest intermittently and often require extended fluoroscopic surveillance to document.
Although videofluoroscopy is a worthwhile diagnostic procedure in these cases, in our experience, intermittent lesions are frequently missed. Various types of hiatal hernias have been reported in dogs and cats, including the type 1 or sliding hernia, in which the gastroesophageal junction lies within the thoracic cavity. In type 2, or paraesophageal hiatal hernia, the gastric fundus or other abdominal viscera are displaced through the hiatus and located in the thoracic cavity, but the gastroesophageal junction is in a normal position. In type 3 or mixed hiatal hernias, characteristics of type 1 and 2 are observed. Type 4 is a type 3 complicated by stomach or other abdominal viscera being located in the paraesophageal sac. The sliding hiatal hernia is the most commonly reported in the Shar pei dog as well as in other dog breeds.
References
1. Watrous BJ. Clinical presentation and diagnosis of dysphagia. Vet Clin North Am Small Anim Pract 1983;13(3):437-459.
2. Watrous BJ, Suter PF. Normal swallowing in the dog: A cinefluorgraphic study. Vet Radiol Ultrasound 1979;20:99-109.
3. Warnock JJ, Marks SL, Pollard R, Kyles AE, Davidson A. Surgical management of cricopharyngeal dysphagia in dogs: 14 cases (1989-2001). J Am Vet Med Assoc 2003;223(10):1462-1468.
4. Pollard RE, Marks SL, Davidson A, Hornof WJ. Quantitative videofluoroscopic evaluation of pharyngeal function in the dog. Vet Radiol Ultrasound 2000;41(5):409-412.
5. Davidson AP, Pollard RE, Bannasch DL, Marks SL, Hornof WJ, Famula TR. Inheritance of cricopharyngeal dysfunction in Golden Retrievers. Am J Vet Res 2004;65(3):344-349.
6. Bright RM, Sackman JE, DeNovo C, Toal C. Hiatal hernia in the dog and cat: a retrospective study of 16 cases. J Amall Anim Pract 1990;31:244-250.
7. Lorinson D, Bright RM. Long-term outcome of medical and surgical treatment of hiatal hernias in dogs and cats: 27 cases (1978-1996). J Am Vet Med Assoc 1998;213:381-384.
8. Pollard RE, Marks SL, Leonard R, Belafsky PC. Preliminary evaluation of the pharyngeal constriction ratio (PCR) for fluoroscopic determination of pharyngeal constriction in dysphagic dogs. Vet Radiol Ultrasound. 2007;48(3):221-6.