Hematologic and Biochemical Methodologies and the Development of Reference Ranges
Kendal E. Harr, DVM
Abstract
“A physician who depends on the laboratory to make his diagnosis is probably inexperienced; one who says that he does not need a laboratory is uninformed. In either instance the patient is in danger.”—JA Halsted
According to the Principles of Quality Assurance authored by the American Society of Veterinary Clinical Pathologists, every laboratory should generate reference ranges for appropriate species for each machine in that laboratory.3 It should be noted that two different machines can and will have different reference ranges. For instance, clinically significant differences are frequently observed between chemistry analyzers that use wet reagents, such as the Hitachi 911, and dry reagents, such as Kodak Ectachem analyzers (Tables 1, 2).
Table 1. Reference values for Hitachi 911 analyzer

Table 2. Reference values for Kodak Ektachem analyzer
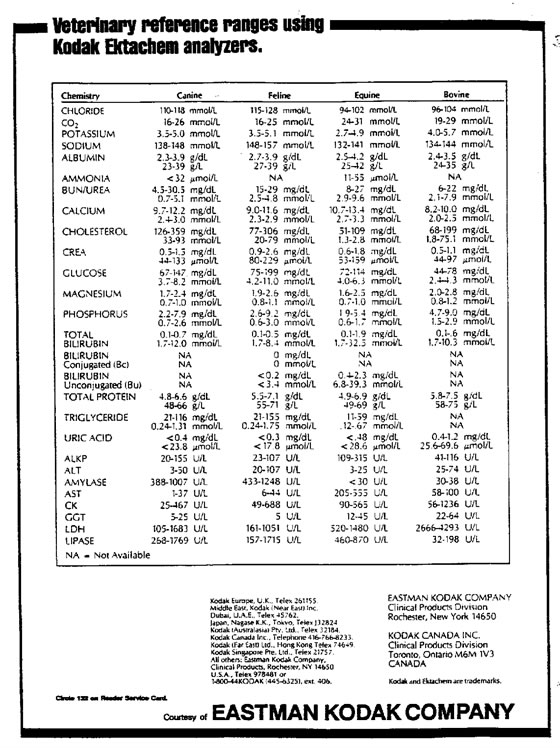
A reference range is obtained by sampling a representative population, statistically eliminating outliers, with the resultant limits defining values equivalent to health. In human medicine, a reference range is generated with no less than 120 clinically healthy individuals. This is not always practical in veterinary medicine. Accepted veterinary protocol generally requires no less than 30, though 40 individuals are preferred.
Most values have a Gaussian or normal distribution and can be evaluated using statistics that follow the rules of normality. Exceptions to this generally include total bilirubin, eosinophils, and basophils. Ninety-five percent of the Gaussian distribution is located within 1.96 standard deviations of the mean. Reference ranges are calculated using 1.96 standard deviations or can be calculated using quartiles for populations with lower numbers of individuals. Using this method, 2.5% of healthy animals will have values above the reference range and 2.5% of healthy animals will have values below the reference range. Therefore, there is a 5% chance that a true normal animal would be classified as having a mild abnormality. Clinicians are willing to designate normal animals with extreme values of a particular analyte as being abnormal as a trade-off for not accepting too many abnormal animals as normal. A clinician cannot determine whether a single measured borderline value represents an abnormality in that individual or is a normal state.
Reference ranges may be inappropriately widened by variation within a population. For instance, neonates and young juveniles of most species are known to have increased phosphorus, decreased blood urea nitrogen, and transient anemia. If these were included in the reference range, many abnormal animals would not be detected.
In an ideal setting, one would develop a baseline on every individual animal and compare this to the established reference range of clinically healthy animals in that laboratory. This can be quite costly and again is not always practical. Therefore, clinicians are frequently dependent on reference ranges that are hopefully generated by the laboratory that they are using. Frequently, when dealing with exotic animals, clinicians are dependent on reference ranges in books developed using unknown methods that may or may not generate relevant numbers.
Reference ranges can be impacted by selection of subclinically ill animals, a poor quality sample such as a hemolyzed or lipemic sample, the methodology used, poor laboratory quality control, and sampling a small number of animals in a population with significant variation.
There are several artifacts that may originate in the animal or be due to sample handling. Lipemia, hemolysis, and icterus can falsely elevate or decrease so many values that moderate to severe change can almost result in random numbers. Severe hyperglycemia can falsely elevate total protein generated on a refractometer and falsely lower potassium on a dry analyte chemistry analyzer. Marked hyperproteinemia can falsely lower electrolytes determined by the flame photometric method. Ketonemia can falsely elevate creatinine and AST. Severe azotemia can falsely elevate total bilirubin, glucose, and total protein on a refractometer. (Further detailed information can be found in references 1 and 7.)
Methodologies may also cause significant variation. Manual white blood cells counts are imprecise and have documented variation of over 30%.4,8,10 This is currently the only commercially available way to generate a white blood cell count for nucleated species. Unfortunately, variation increases with inflammation and therefore trends in white blood cell counts can be difficult to interpret. New hematology analyzers such as the Cell-Dyn 3500 have a new computer design which enables automated white blood cell counts. This has been validated in alligators at the University of Florida. Strict validation procedure and quality control should be performed prior to generating numbers that would be used clinically. In the alligators, we have seen a significant increase in the variation of WBC counts with the anticoagulant heparin. In lambs, Synnove observed an increased variation from 5% using EDTA to 30% using heparin.9 This means that automated counts will only be worthwhile in species that can be collected in EDTA. This includes pythons, parrots, alligators, and iguanas. Further work is needed to determine which species can be collected in EDTA.
Certain enzymes that are measured to assess organ function must be handled very carefully to ensure accurate results. For instance, sorbitol dehydrogenase (SDH) is found in the cytosol of hepatocytes and testicular tissue. It is generally considered liver specific. However, it is very labile in serum, especially at increased temperatures. It must be run within 8 h and preferably sooner. Even when frozen, the serum concentration of this enzyme will decrease by 25% in 1 wk.2 One can imagine how problems with transport could affect this value. For this reason, it has been replaced with ALT in small animals. Additionally, the assay must be performed with strict controls and is one of the more difficult assays to run. It is still used in large animals, especially at referral institutions. However, the clinician should be aware of its limitations and use discretion when interpreting this value with the rest of the chemistry panel and the clinical presentation. When establishing a reference range for SDH, one must have intimate knowledge of sample handling and laboratory quality control to ensure that the reference range is representative. Ammonia levels are useful to assess liver function and hepatic encephalopathy but have similar quality control issues to SDH.
Bile acids can be measured by either the direct enzymatic method that quantifies total serum 3 alpha-hydroxylated bile acids or a radioimmunoassay (RIA) procedure that quantifies specific bile acid moieties. Different RIA kits quantify different bile acid moieties; the most commonly used RIA procedure used in veterinary laboratories measures nonsulfated conjugated primary bile acids. The enzymatic procedure measures all 3 alpha-hydroxylated bile acids whether they are conjugated or not and is a better estimate of total serum bile acid concentrations. The direct enzymatic method is the procedure that has been clinically validated as a diagnostic test in dogs and cats. Note that the direct enzymatic method and RIA quantitate different compounds. One cannot compare numbers generated by these methods as they are actually measuring different compounds in the serum or plasma. If RIA was to be used regularly in small animals, a reference range for RIA would also have to be established in dogs and cats. One advantage of RIA is that it is less affected by hemolysis; however, the assay is only linear up to 50 µmol/L and dilutions are frequently needed. Heparin has been reported to artifactually decrease bile acid specifically using the colorimetric enzymatic reaction.5 Therefore, the RIA is the method that is used for bile acids in birds at the University of Florida. It has not been validated.
There are several different methods that have been employed to measure different hematologic and biochemical values over the years. These methods go in and out of favor in the same way that treatments fall in and out of favor. When measuring total protein, the biuret method is the most common method used in chemistry laboratories while the refractometer is the most common method used in private practices. In the biuret method, cupric ion reacts with the peptide linkages of protein in a basic solution to form a violet-colored complex with an absorption maximum of 540 nm. This has proven to be the most specific method for measuring total protein and is cost effective. A refractometer measures the scatter of light caused by a solution. Highly refractile non-protein compounds, such as glucose, can significantly change the value observed. This method is not as specific and Lumeij and Debruihne demonstrated that it is unreliable in avian practice. The refractometric method consistently yielded higher results than the biuret method, and the correlation coefficient between these two methods was low.6 Including values from both of these methods in a single reference range for total protein would be inappropriate.
Albumin is commonly determined chemically by the bromocresol green dye-binding method. Most commercial laboratories use a human standard for total protein and albumin determinations, without validation for specific species. Discrepancies between values obtained by dye binding techniques and those obtained by electrophoresis have been demonstrated for chicken, duck, turkey, and pigeon.6 Albumin determinations performed with dry methods have not been validated for use in birds or reptiles.
Assuming that sample collection and handling is adequate and the methodologies are consistent, one must then be concerned with quality control in the laboratory. Bulbs on spectrophotometric machines age and decrease in intensity. Additionally, specific wavelengths may not be emitted. Different assays use different wavelengths and so an aging bulb may affect some tests and not others. The optics in a machine can become dirty and affect accurate readings. Kinetic enzyme chemistries are also dependent on temperature, so significant temperature fluctuation in the lab or within the machine can alter results. Daily quality control and regular calibration is required to ensure dependability of results. Of course, adequate technical training of personnel is required to ensure meaningful results in both chemistries and hematologies.
Attempting to establish reference ranges for the numerous species in the field of zoologic medicine is daunting. Strict quality control at all levels is necessary. This should be followed by statistical analysis to ensure that reference ranges are accurate. If practical, each zoologic institution should establish normals within its own laboratory using a specific methodology. Differences between institutions could then be addressed. Continual reassessment of reference ranges is required as methodologies change and more animals are sampled.
Literature Cited
1. Burkhard, M.J. and D.J. Meyer. 1995. Causes and effects of interference with clinical laboratory measurements and examinations. In: Kirk’s Current Veterinary Therapy XII. WB Saunders Co., Philadelphia, Pennsylvania, Pp.15.
2. Horney, B.S., D.J. Honor, A. MacKenzie, and S. J. Burton. 1993. Stability of sorbitol dehydrogenase activity in bovine and equine sera. Vet. Clin. Pathol. 22:5–9.
3. http://www.asvcp.org/asvcppublications.html#QAS (VIN editor: link could not be accessed on 2/25/21).
4. Hubl, W., S. Hauptlorenz, L. Hustos, R. Jilch, M. Fischer, and P.M. Bayer. 1995. Precision and accuracy of monocyte counting. Comparison of two hematology analyzers, the manual differential and flow cytometry. Am. J. Clin. Pathol. 103(2):167–70.
5. Lumeij, J.T. 1991. Fasting and postprandial plasma bile acid concentrations in racing pigeons (Columba livia domestica) and mallards (Anas platyrhynchos). J. Assoc. Avian Vet. 5(4):197–199.
6. Lumeij, J.T. 1998. Avian clinical biochemistry. In: Clinical Biochemistry of Domestic Animals. Academic Press, New York, New York, Pp. 859–861.
7. Meyer, D. and J. Harvey. 1998. Veterinary Laboratory Medicine. WB Saunders Co., Philadelphia, Pennsylvania, Pp. 5.
8. Rappaport, E.S., B. Helbert, R.S. Beissner, and A. Trobridge. 1988. Automated hematology: where we stand. South. Med. J. 81(3):365–370.
9. Synnove, V., T. Framstad, W. Torseteinbo. 2000. Hematologic evaluation of normal and anemic lambs with the Technicon H1 using EDTA or heparin as anticoagulants. Vet. Clin. Pathol. 29(2):62–68.
10. Trinh, M. 1991. White blood cell counts by automated and manual methods with backlighting. Clin. Lab. Sci. 4(1):45–7.