Abstract
Over the past 4 years, immobilizations of South American fur seals (Arctocephalus australis) (FS), South American sea lions (Otaria byronia) (SL), and southern elephant seals (Mirounga leonina) (SES) have been conducted in Peru and Argentina for ecological studies and population health surveys. During the handling procedures, animals were fitted with radio-tracking or behavioral recording devices, individually identified and examined, and blood and fecal samples were collected for analysis.
Three hundred and eleven adult FS males, females and their neonates were examined during three pupping seasons from 1992–1994 at Punta San Juan, Nazca Department, Peru. Females and pups were manually restrained. Male FS were darted with a CO2-powered pistol using 3-ml plastic darts and 1.5x38-mm collared needles (Telinject USA, Saugus, CA, USA). Darting was performed after approaching a territorial male and confirming that the animal would not abandon its territory if disturbed. Most males (n=32) were immobilized using only a premixed combination of equal parts tiletamine hydrochloride and zolazepam (Telazol®, Fort Dodge Laboratories, Fort Dodge, IA, USA) using a mean dosage of 1.43 mg/kg bodyweight. Four males were immobilized using Telazol® but supplemented with 75 or 100 mg IM doses (x̄=0.81 mg/kg) of ketamine hydrochloride (KET) (Ketaset®, Fort Dodge Laboratories, Fort Dodge, IA, USA) when they were not immobilized enough for safe handling. Additional males (n=8) were immobilized with a combination of Telazol® (x̄=1.15 mg/kg) and KET (x̄=0.27 mg/kg) mixed in a single dart. One adult male was immobilized with KET (1 mg/kg) and midazolam (0.1 mg/kg). A benzodiazepine antagonist, flumazenil (Mazicon®, Hoffmann-La Roche, Nutley, NJ, USA), was administered to most FS (n=36) that received Telazol®. For 16 FS, flumazenil (0.3–0.5 mg) was given intramuscularly immediately upon induction of anesthesia (x̄=10.6 minutes after darting) to reverse some of the respiratory depressant effects of the zolazepam component of the Telazol®. For 25 FS, flumazenil (3.0–5.0 mg) was given intramuscularly at the end of the handling procedure to antagonize the effects of zolazepam in animals that were still heavily sedated at the end of the handling procedure.
Most of the SL and all of the SES were handled in Chubut and Santa Cruz provinces of Argentina. For the SL, 19 adult females were immobilized for radio-tagging. Thirteen were immobilized using Telazol® (x̄=2.75 mg/kg) given intramuscularly with a syringe after restraining the animal in a net. Seven SL were immobilized with isoflurane (Ohmeda PPD, Inc., Liberty Corner, NJ, USA) after restraining the animal in a net. Atropine hydrochloride (0.02 mg/kg) was given intramuscularly 5 minutes prior to anesthetic administration for seven of the FS receiving Telazol® and the five receiving isoflurane. Flumazenil was given intramuscularly to females immobilized with Telazol® at the end of procedures using 1 mg of flumazenil for every 20–25 mg of zolazepam administered. Sea lion pups were manually restrained for tagging, examination, and sample collection while their dams were immobilized. Elephant seals (n=4) were immobilized with Telazol® (0.6–1.7 mg/kg) given intramuscularly while they were resting or sleeping on the beach.
Immediately upon capture, each animal received a physical examination. A fecal sample was collected manually from the rectum when possible and preserved in 10% formalin. Blood was collected from a tarsal vein in the adult FS and SL or the dorsal vertebral vein/sinus in the neonates and the adult SES and kept on ice until processing.
Two FS were fitted with standard VHF radio transmitters (Advanced Telemetry Systems, Inc., Isanti, MN, USA). Sea lions were fitted with transmitters utilizing the ARGOS satellite system and containing instrumentation to record data on diving patterns and behavior (Wildlife Computers, Woodinville, WA, USA). Elephant seals were fitted with devices that recorded diving behavior but had to be recovered from the animal to download the stored data (Wildlife Computers, Woodinville, WA, USA). Quick-setting epoxy was used to attach all transmitters and recording devices to the pelage of the animal. The devices then fell off at the time of the next molt or were removed at a later date using manual restraint.
Blood smears, PCV and plasma total solid measurements, and white blood cell counts were all performed in the field. Plasma and serum were frozen in liquid nitrogen or initially frozen at -20°C and later stored at -70°C until laboratory analysis.
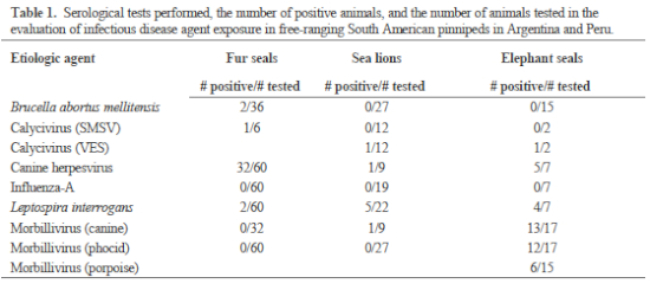
Blood smears were stained and microscopically evaluated to detect the presence of blood parasites and to perform white blood cell differentials. Serum chemistries and enzymes were processed on an automated analyzer. Plasma samples were analyzed for vitamin E (α-tocopherol) and vitamin A (retinol) using high-performance liquid chromatography. Plasma samples were analyzed for aluminum, boron, barium, copper, cobalt, iron, magnesium, manganese, molybdenum, and zinc by inductively coupled argon plasma emission spectroscopy. Plasma was also analyzed for polychlorinated biphenyls and the following chlorinated pesticides: aldrin; alpha-BHC; beta-BHC; O, P’-DDD; P, P’-DDD; P, P’-DDE; O, P’-DDT; P, P’-DDT; dieldrin; endrin; heptachlor; heptachlor epoxide; and Lindane (gamma-BHC). Infectious disease serology tests are listed in Table 1. Fecal samples were examined using direct microscopic examination, sodium nitrate flotation, and sedimentation methods. Eleven SL yearlings and three FS yearlings were found dead during the non-breeding season, and tissues were collected for histopathology.
Mean time to achieve maximum effect was 10.6 minutes for Telazol® in the male FS. For the Telazol® and KET combination, it was 10.0 minutes. For Telazol® in the female SL it was 10.7 minutes, and in the SES it was 10.0 minutes. Telazol® provided predictable and adequate immobilizations in almost all animals for the handling procedures conducted. Respiratory depression associated with benzodiazepines was seen in the FS and SL, but the use of low doses (0.05–1 mg) of flumazenil rapidly improved depth of respiration during the procedures. For complete reversal of benzodiazepines, the recommended dosage of flumazenil is 1 mg for every 20–25 mg of benzodiazepine used. The flumazenil appeared to eliminate the “drunken” appearance of animals recovering from anesthesia, and it increased alertness and responsiveness within minutes of administration.
Isoflurane resulted in complete immobilization in a mean of 11.7 minutes. Recovery from isoflurane anesthesia was rapid (3–5 minutes) and complete.
Blood parasites or ectoparasites were not seen in any of the animals sampled. Ectoparasites or signs of parasitic dermatitis were not observed on any animal. Ascarid ova were found in one of the seven fecal samples from SL. Of the 19 samples from FS, five had strongyle-like ova and two had trematode ova.
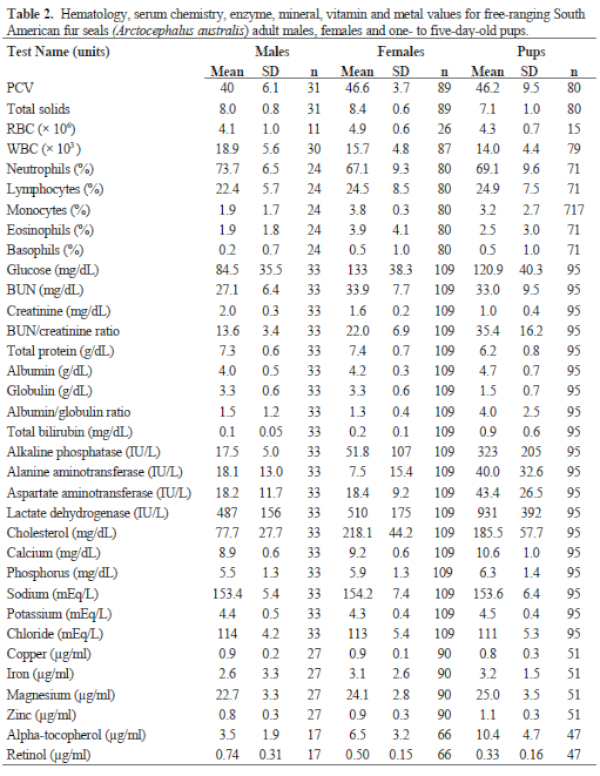
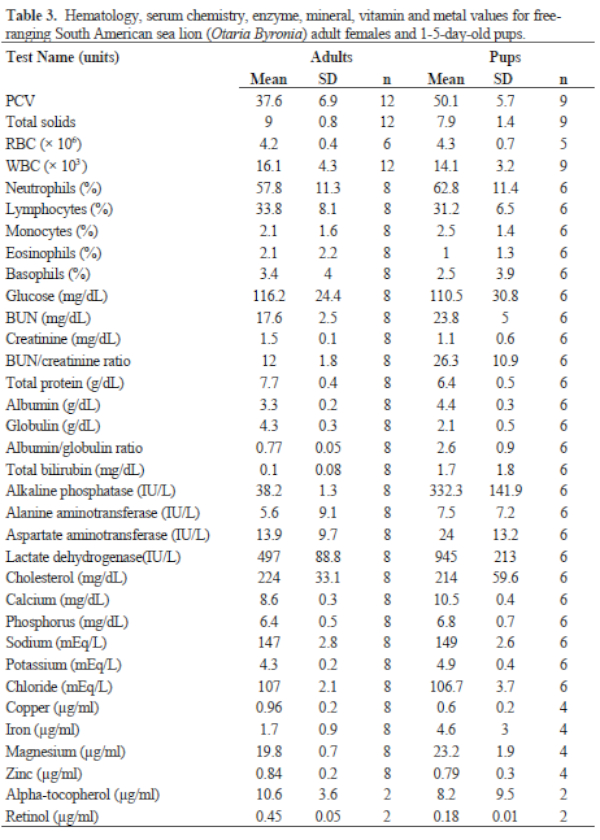
Results of FS serum chemistries, enzymes, and vitamin and mineral analyses for the sex and age classes are provided in Table 2. Results for SL are in Table 3. Levels for Al (<1.66 ppm), B (<1.66 ppm), Co (<0.167 ppm), Mn (<0.083 ppm), and Mo (<0.33 ppm) were below accurate detectable limits. All chlorinated pesticide and polychlorinated biphenyl analyses were below detectable limits (0.001–0.007 ppm and 0.05 ppm, respectively). Serology results are in Table 3.
Histopathology from the yearlings showed lymphoplasmacytic enteritis in nine of the 11 SL, with multifocal crypt abscessation in seven, and one of the three FS also was found to have lymphoplasmacytic enteritis.