Nutrition and Medical Management in the Critically Ill Patient with Gastrointestinal Disease
Introduction
Gastrointestinal disease is very common in small animal veterinary patients; in many patients, it is mild and self-limiting and does not need specific treatment; however, others may be critically ill. As well as pursuing a diagnostic workup, these patients typically need supportive care during their workup and definitive management. In this lecture, we will explore the medical and nutritional management of patients with severe GI disease based on a case discussion.
Fluid Therapy in the Critically Ill Patient with GI Disease
Large volumes of fluid and electrolytes are secreted and reabsorbed by the healthy GI tract daily. In a 20-kg dog, approximately 2.5 L of fluid enter the GI tract from diet and normal gastrointestinal secretions and over 98% is reabsorbed daily. Of the fluid entering the GI tract, approximately one quarter to one third is from external sources (food and water) and the majority from endogenous secretions (salivary glands, stomach, small intestine, liver, and pancreas). The nature of the fluid secreted at different points along the GI tract is quite variable and so the systemic derangements are also very variable depending on the exact nature of the disease. Furthermore, significant fluid losses into the GI tract commonly lead to systemic fluid imbalance (hypovolaemia and/or dehydration) with further implications for electrolyte homeostasis.1,2 Both hypovolaemia and dehydration are frequently present to some degree on presentation. Furthermore, during the course of the disease, fluid losses are difficult to predict and it is not uncommon for patient to have one or more periods of hypovolaemia that are addressed and then redevelop. As some of the critically ill patients are puppies (e.g., parvovirus), assessment of both hydration and perfusion can be more challenging than in the adult animal. Generally, an isotonic balanced electrolyte solution is the first fluid of choice (e.g., Hartmann's solution, lactated Ringer's solution or 0.9%NaCl). Aggressive fluid rates may be necessary and initially, up to 90 ml/kg can be given and the response assessed. In the more severely affected patients with hypoalbuminaemia, vasculitis and/or septic shock, response to crystalloid therapy can be disappointing and colloid therapy is required. Similarly, bolus doses of up to 20 ml/kg may be used acutely to correct volume status. Once the hypovolaemia is corrected, a longer-term crystalloid fluid plan consisting of estimated maintenance, replacement of hydration and ongoing losses can be calculated. It must be remembered though that this "ongoing support" figure is very much a best guess and patient should be monitored closely, at a minimum every 4 hours in early stages, and sometimes more frequently. Despite our best guess, a high proportion of these patients will redevelop signs of poor perfusion at some point; prognosis will be better if this is identified at the earliest possible stage and bolus doses of either crystalloid or colloid repeated. Transfusion therapy may also be useful in selected patients; plasma can be used largely for its clotting factor content in cases with prolonged enteral bleeding or DIC, and whole blood is an option if the puppy is anaemic. The albumin found in plasma or whole blood is unlikely to be of much benefit.
Specific Electrolyte Changes
Sodium
Patients with gastrointestinal disease may have low, normal, or high serum sodium at the time of presentation. This is dependent on the exact nature of the GI tract losses (i.e., whether they contain more sodium than water or vice versa) and the ability of the animal to drink and regain free water. Hyponatraemia may be quite dramatic in patients that have had some isotonic fluid loss through the GI tract, leading to hypovolaemia and stimulation of ADH secretion and thirst. If the patient has access to free water and is able to drink and absorb it within GI tract, then the kidneys under the influence of ADH will act to retain water; this occurs even in the face of ongoing hyponatraemia (and hypo-osmolality) as the baroreceptor influence on ADH secretion overrides the osmoreceptor influence.
Potassium
Similarly to sodium, patients with gastrointestinal disease may have low, normal, or high potassium at the time of presentation. As large amounts of potassium are secreted both into the stomach and small intestine on a daily basis, hypokalaemia is common and may be severe. Hyperkalaemia (and especially significant hyperkalaemia) is uncommon with primary GI disease and should raise the suspicion of a systemic cause for the electrolyte disturbance and the clinical signs.
Chloride
Hypochloraemia is a common finding in patients with vomiting; it is a factor in perpetuation of metabolic alkalosis (see above) and should be corrected when identified.
Calcium
Ionised hypocalcaemia may be seen with both severe small intestinal disease (specifically lymphangiectasia) and acute pancreatitis. The mechanism is unknown. Possibilities include calcium/fatty acid deposits being retained in the GI tract lumen and reducing calcium absorption, hypovitaminosis D from malabsorption and/or hypomagnesaemia affecting PTH secretion. Although clinical signs secondary to hypocalcaemia are rare they may occur.3 Hypocalcaemia has been found to be a negative prognostic indicator in cats with acute pancreatitis. Total calcium may be low in a number of patients with gastrointestinal disease and hypoproteinaemia; wherever possible, an ionised calcium should be measured to understand the physiological significance of this.
Nutritional Evaluation of the Critically Ill Gastrointestinal Patient: When to Intervene
All critically ill patients should undergo a nutritional evaluation4 to establish a nutritional plan, including a physical examination with body weight, body condition score (BCS), and muscle condition score. Very old and very young patients are more prone to malnutrition than adults. A history of decreased food intake or anorexia, clinical signs associated with nutrient and energy losses (vomiting, diarrhea), and involuntary weight loss are also important considerations. Blood work is neither sensitive nor specific. The recommendation is to start feeding as soon as the patient is hemodynamically stable. One study5 in dogs with parvovirosis showed that enteral feeding within 12 hours of admission resulted in better outcome than waiting 12 hours until vomiting had subsided to start feeding. Nutritional intervention is required in patients after 3–5 days of hypo/anorexia (3 days maximum in high-risk patients), and if there is loss of more than 10% body weight.
Selecting Feeding Route
| Figure 1. Algorithm for assisted feeding in critically ill gastrointestinal patients | 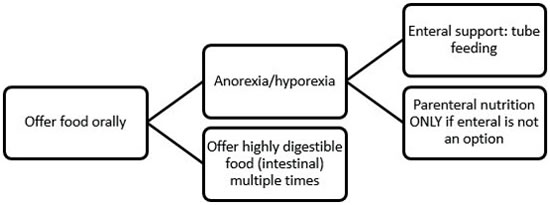
|
|
| |
All efforts should be made to stimulate voluntary food intake as soon as possible.6 Use different textures and flavors, human foods, warming up the food, having the owner feed the patient, etc. If still not eating, assisted feeding is indicated. Historically, patients with gastrointestinal (GI) disease have been subjected to fasting or parenteral nutrition, but recent research shows that early enteral feeding can be beneficial. Thus, a nasoenteral feeding tube can be placed (or even esophageal feeding tubes, if the patient is stable enough for a short anesthesia).
Energy Requirements
The conservative recommendation is to use resting energy requirements (RER) calculated as 70 x Body weight (kg)0.75 in kcal per day. Adjustments can be done every 48 hours to maintain a stable body weight.
Diet
Highly digestible intestinal diets are recommended to promote maximum digestion and absorption of nutrients. In patients with feeding tubes, these can be used as well, except for NE tubes, where only liquid diets can be used to provide enough calories. Convalescence-type diets can also be used if the patient tolerates their high fat content.
Feeding Method
Multiple feedings a day (4–6) are indicated. Constant rate infusion can be used if the patient is volume intolerant. The first day, 1/3 of RER should be provided and increased to 100% over 2–3 days. If there are signs of intolerance (vomiting, abdominal pain, signs of nausea), consider the need to change the diet to something more energy dense or increase the number of feedings per day or take one more day to reach RER.
References
1. Boag AK, Coe RJ, Martinez TA, Hughes D. Acid-base and electrolyte abnormalities in dogs with gastrointestinal foreign bodies. J Vet Intern Med. 2005;19(6):816–821.
2. Heald RD, Jones BD, Schmidt DA. Blood gas and electrolyte concentrations in canine parvoviral enteritis. J Am Anim Hosp Assoc. 1986;22:745–748.
3. Kimmel SE, Waddell LS, Michel KE. Hypomagnesemia and hypocalcemia associated with protein-losing enteropathy in Yorkshire terriers: five cases (1992–1998). J Am Vet Med Assoc. 2000;217(5):703–706.
4. Freeman L, Becvarova I, Cave N, MacKay C, Nguyen P, Rama B, Takashima G, Tiffin R, van Beukelen P, Yathiraj S. WSAVA Nutritional Assessment Guidelines. Compend Contin Educ Vet. 2011;33:E1–9.
5. Mohr AJ, Leisewitz AL, Jacobson LS, Steiner JM, Ruaux CG, Williams DA. Effect of early enteral nutrition on intestinal permeability, intestinal protein loss, and outcome in dogs with severe parvoviral enteritis. J Vet Intern Med. 2003;17:791–798.
6. Delaney SJ. Management of anorexia in dogs and cats. Vet Clin North Am Small Anim Pract. 2006;36:1243–1249.