Chair for Fish Diseases and Fisheries Biology, Faculty of Veterinary Medicine, Ludwig-Maximilians-Universität München, Kaulbachstr, Germany
Diversity of over 30,000 finfish species, and many more other aquatic animals makes a veterinary job rather complex, but only until we realize that as veterinarians, we have been trained in application of common principles of medicine to any animal species, including thousands of species of ornamental fish. There are ecosystem extremes, of course, such as Death Valley pupfish, or Antarctica icefish, and variability in sizes from < 1 cm-long adult killifish to 10+ m of whale shark. But, the common denominator for every finfish is that they live in water, and that water quality is the single most important factor in fish health.
Fish show very few specific signs of illness, making it difficult to make a diagnosis by clinical examination alone. Water quality analysis of several basic parameters such as salinity, temperature, pH, dissolved oxygen (DO), nitrogen compounds (ammonia, nitrite and nitrate) can therefore point a clinician in a good direction, especially since over 80% of clinical signs are connected to water quality problems. Therefore, in this presentation, we will focus on water quality parameters that are most commonly causing health problems in ornamental fish.
Generally, freshwater fish will not survive for long in seawater, and vice versa. This may be too simplified, but it is important to know if a fish you examine normally swims in the ocean or a river. Some novice aquarists may not know the difference or may be ill advised by a dealer. Unfortunately, when exposed to wrong salinity, fish metabolism can't cope with osmotic pressure, and death is imminent within minutes. Such outcome is usually predated by increased breathing rates (rapid opercular/gill cover/movements), exophthalmia, brief excitation followed by lethargy and death. Unfortunately, as mentioned above, these would also be signs of multiple other water quality problems such as low or high pH, acute chlorine or ammonia toxicity, etc. So taking good history is important, as well as looking at all available water quality parameters.
Ornamental fish mostly come from tropical parts of the world, with low fluctuations of water temperatures (26–28°C), but there are also examples of cool water aquarium fish (goldfish, optimal 18–20°C). In general, sudden changes in temperature (faster than 1°C per hour) even for a short time (< day) can cause severe stress and subsequent immunosuppression. Few days or up to a couple of weeks after such stress, an outbreak of facultative pathogens, most commonly fungal (Saprolegnia) or bacterial (Aeromonas spp.) can be observed. Long exposures to suboptimal temperatures will decrease fish ability to cope with the environment and lead to exhaustion and death. Commonly, fish outside of their optimal temperature range will stop eating, and after several days (or even weeks) will become lethargic and start showing signs of opportunistic fungal or bacterial infections (fin rot, whitish cottony patches, petechial hemorrhages, etc.). However, acclimated fish can live for a long time in slightly lower or higher temperatures, and the only consequence may be changes in normal behavior patterns; e.g., lack of spawning, or schooling fish such as neon tetras may seek shelter in cooler areas of an aquarium instead of swimming in a water column.
Dissolved oxygen (DO) is essential for fish survival. Fish suffocate when DO levels are low, and this is to some extent species specific, but as a basic guideline, DO levels less than 5 mg/L are dangerous for most fish. The oxygen carrying capacity of water is affected by the temperature (low levels in warmer waters) and specific gravity (e.g., more salts = less ability to hold DO). Other oxygen-consuming processes such as biological filtration, excess waste mineralization, plant respiration during the night, etc., can further reduce DO available for fish breathing and cause acute depletion. Fish congregate near water inlets and start "piping" in an effort not to gasp air as commonly misinterpreted, but the thin layer of surface water that is in direct contact with air and therefore has more DO. Larger fish are less tolerant of low DO and are usually the ones that die first. Fish that die from low water oxygenation, carbon dioxide toxicity or with gill disease will show gaping mouths - resembling suffocation signs in terrestrial animals.
While carbon dioxide (CO2) is essential for healthy plant growth and is usually present as dissolved gas, or part of bicarbonate buffer, it is advisable to keep it under 6 mg/L because of potential negative (mostly chronic) effects on fish health. Acute CO2 poisoning resembles narcosis in fish and can result in death. Another dissolved gas-related health issue is gas super saturation, most frequently related to nitrogen gas, but also observed with oxygen and other gases. Simply put, the drop in pressure from the municipal supply causes gas in excess of 100% to exit the solution and form bubbles. Such bubbles also form within the fish body, causing gas embolism and related clinical signs such as exophthalmia, visible bubbles in fins and skin, infarcts in gills, sometimes petechial bleeding and finally death.
Most fish thrive when acidity or basic properties of the water (measured as pH value) are in the range between 6 and 8. Freshwater fish are more inclined to neutral to slightly acidic (6.5–7.2) pH values, while marine species prefer pH of about 8.2–8.5. But, species variations are known. The pH level is intertwined with numerous metabolic processes and affects all other water quality parameters, such as solubility of metals (e.g., from sediment, and their potential toxicity), or ionized vs. non-ionized (e.g., more or less toxic) forms of different nitrogen compounds. However, direct effects of the wrong pH are most frequently observed as irritation and erratic behavior of the fish. As with temperature, sudden changes in pH are not well tolerated and can cause irritated fish to jump out of the open tanks. Longer exposures to high or low pH can present as gill irritation (burn), increase in thickness of gill and skin mucus layer that can also become cloudy instead of transparent. Neurological symptoms, such as hyperexcitability, or color pattern changes to dark or pale are also frequently observed in fish exposed to extreme pH values (< 6 or > 9).
Nitrogen compounds (ammonia, nitrite, and nitrate) can be very toxic to fish in increased concentrations (Figure 1). Fish waste is excreted as ammonia that can take two forms (NH3 or un-ionized, and much more toxic; and NH4+ - ionized) depending on pH and temperature (Table 1). Clinical signs of ammonia poisoning include increased mucus production, red or bleeding gills, darkening of body coloration, 'gasping' for air at the surface and increased respiration rate.
| Figure 1 | 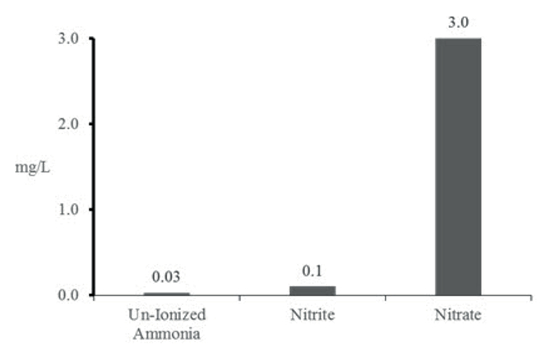
Relative toxicity of different nitrogen compounds. Acute toxicity of each compound occurs if listed levels are exceeded; however, any concentration of un-ionized ammonia will cause chronic toxicity (adapted from Morris 2001). |
|
| |
Table 1. Toxicity of ammonia at different pH in freshwater
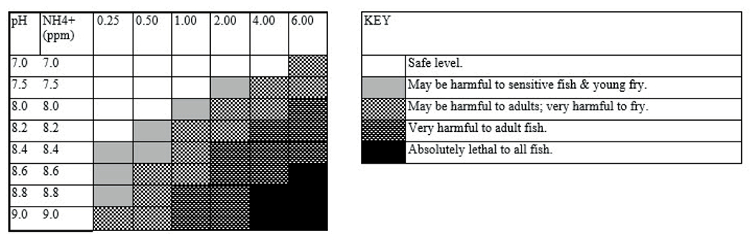
Table sourced from the Hagen water test kit and adopted from Loh (2011).
Total ammonia nitrogen enters the bacterial oxidation cycle in biological filters, producing nitrites (NO2-) followed by nitrates (NO3-). Elevated nitrite levels often occur during the early stages of aquarium setup due to lack of nitrifying bacteria in the filtration system. However, a sudden nitrite spike can suggest an imbalance in the system. Something as simple as washing the filtration media vigorously can remove ammonia nitrifying bacterial population, and antibiotic treatments can kill nitrifying bacteria. Nitrite poisoning is also known as "brown blood disease" because excess nitrite competes for oxygen binding domains in the hemoglobin molecule and gives blood (and gills) characteristic brown color instead of bright red. Other clinical signs are resembling hypoxia (gasping/piping for the air at the surface, lethargy and hyperexcitability, as well as color change). The nitrite toxicity is pH and temperature dependent, similarly to ammonia, but marine fish and juveniles appear to be more sensitive.
Nitrate levels take some time to build up, but increased nitrate concentration can create considerable chronic stress conditions to fish resulting in retarded growth rate, reduced disease resistance and delayed wound healing. Excess nitrate can cause redness in the fins or body most likely due to dilation of blood vessels, mostly notable in white-colored fish.
Finally, many municipalities use chlorine (Cl2) or chloramines to disinfect tap water. Unlike mammals, both are highly toxic to fish, causing gill and skin irritation, followed by severe gill necrosis, suffocation and death. But caution needs to be exercised since use of chemical neutralizers such as sodium thiosulphate can deplete DO and further exacerbate hypoxia in fish with already damaged gills.
References
1. Loh R, Landos M. Fish Vetting Essentials. Richmond Loh Publishing; 2011.
2. Morris JE. Managing Iowa fisheries: water quality. Iowa State University Extension Publication Number PM1352a; 2001.
3. Noga EJ. Fish Disease: Diagnosis and Treatment. John Wiley & Sons; 2011.
4. Roberts HE, ed. Fundamentals of Ornamental Fish Health. John Wiley & Sons; 2011.
5. Wildgoose WH. BSAVA Manual of Ornamental Fish. Gloucester: British Small Animal Veterinary Association; 2001.