Abstract
An outbreak of aspergillosis occurred in the Woodland Park Zoo’s flock of Humboldt penguins. The outbreak was thought to be related to changes in keepers, cleaning procedures, and fish quality. After several deaths, a program of intensive treatment and monitoring was begun. Penguins were treated with itraconazole, multiple antibiotic regimes, fluids, and tube feedings. Monitoring was accomplished with complete blood counts, clinical chemistries, serum protein electrophoresis, and Aspergillus levels. White blood cell counts, Aspergillus levels, and electrophoretic albumin levels were helpful in diagnosis and in monitoring treatment success. Sick birds tended to have high white blood cell counts, high Aspergillus-antibody levels, and/or low albumin values. As birds improved, their white blood cell counts and Aspergillus-antibody levels decreased and their albumin levels increased. Very low albumin levels did not indicate a poor prognosis in this study.
Introduction
Penguins are extremely susceptible to Aspergillus infections and mortality from these infections is often high.1,2,4,5 Antemortem diagnosis of aspergillosis can be difficult but several authors have reported on the usefulness of Aspergillus-antibody levels and serum protein electrophoresis (SPE) in identifying affected birds.3,5 In addition, albumin and globulin levels determined by electrophoresis have shown some promise as prognostic indicators.5
An outbreak of aspergillosis and suspected secondary bacterial infections occurred in the Woodland Park Zoo’s flock of Humboldt penguins (Spheniscus humboldti). Only one bird died of confirmed aspergillosis between 1987 and 1995. However, six birds died of aspergillosis between March 1995 and September 1996. In September 1996 one bird died and five others were noted to be clinically ill, and a program of intensive monitoring and treatment was begun with the remaining birds in the flock. The goals of this program were to use complete blood counts, clinical chemistries, Aspergillus levels, and SPE in an attempt to (1) rapidly detect clinical cases, (2) effectively monitor treatment in birds who became ill, and (3) to further evaluate the efficacy of SPE and Aspergillus-antibody levels as diagnostic and prognostic tools.
Methods
At the beginning of the study period the 13 birds in the flock were bled for complete blood counts, clinical chemistries, Aspergillus-antibody levels, and SPE. Serum protein electrophoresis was performed by Phoenix Central Laboratory in Everett, WA, in a process that has been described by other authors.3 Aspergillus-antibody levels were determined by The Raptor Center at the University of Minnesota (St Paul, MN) using an ELISA test. Reported numbers are optical density readings, with 0.12 being the minimal threshold for a positive result. Because serial dilutions are not performed, these values are not considered titers (Patrick T. Redig, personal communication).
All birds were vaccinated with an Aspergillus vaccine (Willamette laboratories, Portland, OR)6 and placed on oral itraconazole (Sporanox™, Janssen Pharmaceutica, Inc., Titusville, NJ) at a dosage of 10 mg/kg SID. Sick birds were placed on broad-spectrum injectable antibiotics and other supportive treatments (SQ fluids, tube feeding, etc.) as needed. Serial white blood cell counts, Aspergillus-antibody levels, SPE, and clinical impressions were used to evaluate treatment protocols. In addition, a computerized axial tomography (CAT) scan was performed under isoflurane anesthesia on the sickest bird (bird 1) to determine the extent of pulmonary and air sac involvement. A postmortem CAT scan on a confirmed Aspergillus-negative bird (bird 9) was used for comparison.
Blood work was repeated on all birds after six months to reevaluate the health of the flock. Trends in white blood cell counts, protein electrophoretic data, and antibody levels were evaluated using correlation coefficients and scattergrams. These coefficients and scattergrams were generated with Statview 4.02 on an Apple Macintosh computer.
Results
Initial evaluation of the 13 birds in the flock revealed that five birds had positive Aspergillus-antibody levels (birds 1, 3, 6, 7, 8) ranging in value from 0.135 to 0.248. Five birds (birds 1–5), only two of which had positive levels (birds 1 and 3) on initial evaluation, were clinically ill at that time. Birds 2 and 5 developed positive antibody tests within the next month. Early clinical signs of aspergillosis in our penguins included lethargy, decreased swimming time, and dehydration. Later signs included a decrease in appetite, regurgitation, gaping, sitting back on the tail instead of upright, and in very late stages standing stooped over. The sick birds had an average white blood cell count of 27,400 cells/ml3 and an average albumin of 1.61 g/dL. The eight healthier birds, three of which had positive Aspergillus levels, had an average white blood cell count of 15,800 cells/ml3 and an average albumin of 2.33 g/dL.
Three (birds 3, 4, 5) of the five sick birds were treated intensively for three months, then taken off antibiotics and continued to do well. The other two birds (birds 1 and 2) remained on intensive treatment for an additional two months.
During this time, one bird who was not ill (bird 9), fought with another bird, became septic and died from severe gout. No Aspergillus lesions were seen on postmortem examination and cultures were negative. In addition, one of the previously healthy birds (bird 6), who had initially had a positive Aspergillus test became clinically ill with aspergillosis, just as birds 1 and 2 were improving.
When blood analyses were repeated at six months, bird 6 was the only sick bird. Two birds (1 and 6) had positive Aspergillus-antibody levels of 0.181 and 0.176, respectively. The flock had an average white blood cell count of 20,277 cells/ml3, an average albumin of 2.77 g/dL, and an average Aspergillus level of 0.115.
Serial measurements in the five original sick birds revealed a general decrease in Aspergillus-antibody level and total white blood cell counts with a general increase in albumin level as the birds improved clinically (Figure 1). Although albumin levels tended to increase and total white blood cell counts tended to decrease as Aspergillus-antibody levels decreased, the correlations between these variables were not statistically significant. Albumin levels were lowest in birds who were doing poorly and tended to increase as birds became healthier.
Figure 1
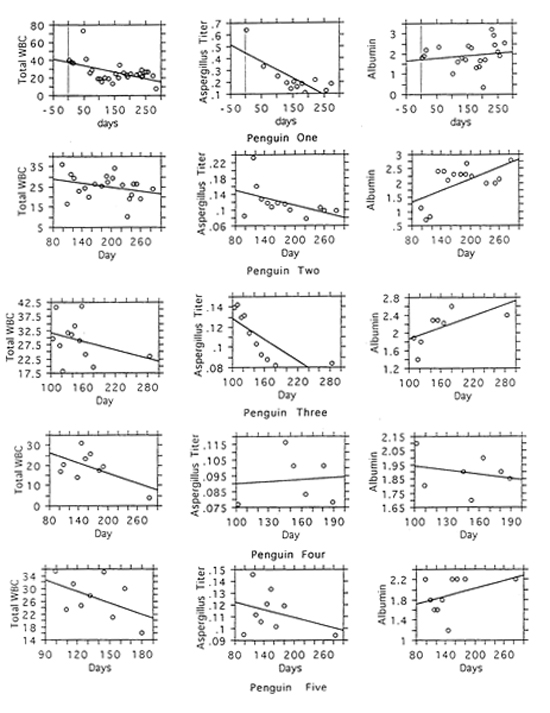
Changes in white blood cells, Aspergillus titer, and albumin levels over time
A CAT scan done on bird 1 revealed lesions in the cranial thoracic air sacs that suggested fungal infiltration. These lesions were visualized as multi-septated planes of soft-tissue within the air sacs. No lesions were seen in the lungs.
Discussion
Changes in keeper coverage and cleaning procedures in the unit are thought to have contributed to this outbreak. In particular, the excessive use of bleach in cleaning the penguin pool is thought to have been an important stressor. The use of new keepers in the area who were less familiar with penguin behavior may have delayed rapid identification of sick birds and increased the initial mortality rate. Finally, poor fish quality was also a contributing factor. The severity of this outbreak and the expense of treatment reiterates the importance of careful evaluation of changes in routine when dealing with penguins. In addition, having keepers who can recognize early clinical signs of illness and who are willing to aggressively pursue fluid and nutritional therapy is crucial in bringing an outbreak under control.
Since the program of intensive monitoring and treatment began, only one bird has died, and this death was not attributable to aspergillosis. In addition, the health of the whole flock has improved as evidenced by a decrease in the number of sick birds (five to one), a decrease in the average total white blood cell count, an increase in the average albumin level, and a decrease in the number of birds with positive levels (five to two).
Serum protein electrophoresis did give important diagnostic information. Three of the original five sick birds (birds 2, 4, and 5) strongly suspected to have aspergillosis based on clinical signs and high white blood cell counts, had negative Aspergillus levels. However, these birds did have low albumin levels. These birds are suspected to have had negative levels and low albumin levels due to immunosuppression. This is consistent with Reidarson’s report of birds confirmed with aspergillosis on postmortem examination that had negative levels. Two of our birds (2 and 5) did develop positive Aspergillus levels later in the disease course, possibly when they were improving enough to mount an immune response.
Single SPE data points did not appear to give reliable prognostic information. In Reidarson’s report,5 27 of 29 birds with albumin values less than 1.8 g/dL died.5 In contrast, all six of our sick birds had albumin values less than or equal to 1.8 g/dL at some point in the disease. These albumin values then increased as the birds were treated. One explanation for this difference in results may be the intensive treatment that our very sick birds received.
Although total white blood cell count, albumin level, and antibody levels all give important clinical information, a significant correlation is not present among these variables. Thus, none of these tests can be used to predict values in the other tests (e.g., animals with low albumin values may not show high white blood cell counts). Therefore, diagnosis is likely to be most accurate when using all three tests. In our flock, for example, it was important to treat birds who had some clinical signs of aspergillosis, elevated white blood cell counts, and decreased albumin levels even when they had negative Aspergillus-antibody tests.
Because immunosuppression is a feature of aspergillosis, birds are susceptible to secondary bacterial infections. Some of the elevations in white blood cell count seen in our birds may have been due to these infections as white blood cell counts were somewhat responsive to antibiotics. Deep tracheal cultures were not performed in our birds due to the stressful nature of this procedure, so it is difficult to confirm the presumptive joint contributions of fungal and bacterial pathogens.
CAT scans, although not widely accessible, can give useful information about the extent of fungal infiltration of the air sacs. The lesions seen in bird 1’s scan would not have been apparent on radiographs. Location of lesions can be accurately determined, which is important if considering surgical debulking of fungal plaques. Thus, this may be a useful tool when available. Difficulties with this procedure include the need for anesthesia and the need for the bird to be recumbent during the scan. In addition, since lesions can be subtle, scans need to be interpreted by someone familiar both with CAT scans and with bird anatomy.
Acknowledgments
The authors would like to thank Linda Shipe, Harmony Frazier, and Carol Simkins for help with sample collection and laboratory analysis, and Susan Walls for help with preparation of this document. We would also like to thank Dr. Robert Liddell, who performed and evaluated the CAT scan on bird 1. Finally, we would like to thank keepers Allison Barr, Paul Cowell, Ericka Harris, and Tina Mullet whose intensive care of these birds helped in their recovery and survival.
Literature Cited
1. Aguilar RF, Redig PT. Diagnosis and Treatment of Avian Aspergillosis. In: Kirk’s Current Veterinary Therapy XII: Small Animal Practice. Philadelphia, PA: WB Saunders; 1995:1294–1298.
2. Bauck L. 1994. Mycoses. In: Avian Medicine: Principles and Application. Lake Worth, FL: Wingers Publishing, Inc.; 1995:997–1006.
3. Cray C, et al. Plasma protein electrophoresis: principles and diagnosis of infectious disease. In: Proceedings from the Association of Avian Veterinarians. 1995:55–59.
4. Redig PT. Avian Aspergillosis. In: Zoo and Wildlife Medicine: Current Therapy III. Philadelphia, PA: WB Saunders; 1993:178-181.
5. Reidarson TH, et al. Serum protein electrophoresis and Aspergillus-antibody levels as an aid to diagnosis of aspergillosis in penguins. In: Proceedings from the Association of Avian Veterinarians. 1995:61–64.
6. Yearout DR. Prevention and treatment of aspergillosis by vaccination: a new protocol. In: Proceedings from the Association of Avian Veterinarians. 1988:139–143.