Pharmacokinetics of Enrofloxacin After Oral and Intramuscular Administration in Savanna Monitors (Varanus exanthematicus)
Abstract
Introduction
Enrofloxacin is usually given subcutaneously or intramuscularly to reptiles at a dosage of 2.5–5.0 mg/kg at intervals varying from SID to once every 5 days. Adverse effects reported for injectable enrofloxacin include muscle soreness and local tissue swelling after injection.5 Oral administration of enrofloxacin has recently become popular, although most dosages for oral enrofloxacin in reptiles are empirically derived. If absorbed, orally administered enrofloxacin could prove useful for treatment of very large or very small reptile patients.
Methods
All protocols were approved by the Institutional Animal Care and Use Committee at the National Zoological Park, Washington, DC. Ten adult savanna monitors (Varanus exanthematicus), weighing from 1.2 kg to 2.0 kg, were used from the Department of Zoological Research at the National Zoological Park in Washington, DC. The lizards were individually housed (86 × 71 × 58 cm cages), and maintained at 27 ± 1°C. They were maintained on a diet of whole laboratory mice offered once/week. The lizards were assessed to be clinically healthy 2 weeks prior to the study based upon physical exam, survey radiographs, and routine blood analyses (complete blood count and chemistry panel). The pre-trial blood sample was also used as the “zero sampling” time for administration of the antibiotics. Plasma concentrations of enrofloxacin and its metabolite, ciprofloxacin, were determined by high-performance liquid chromatography (HPLC).1 The detection limit of quantitation of enrofloxacin and ciprofloxacin using this method was 0.05 µg/ml.
In the first trial, 10 savanna monitors were each fed a laboratory mouse containing 10 mg/kg of injectable (22.7 mg/ml) enrofloxacin (Baytril, Miles Inc., Shawnee Mission, KS, USA). Each lizard was manually restrained for collection of 1 ml of heparinized blood from the caudal tail vein at predetermined intervals following ingestion. Five animals were sampled four times (30 minutes, 3 hours, 12 hours, and 36 hours) and five were sampled three times (1 hour, 6 hours, and 24 hours).
Two weeks later in the second trial, 10 savanna monitors were manually restrained and given an intramuscular injection of enrofloxacin at a dosage of 10 mg/kg in the left forelimb. One milliliter of heparinized blood was collected from the caudal tail vein at the time intervals described in Trial 1.
Results
The highest measured mean plasma concentration of enrofloxacin was 5.82 µg/ml at 36 hours post-ingestion (Fig. 1). The peak plasma concentration of enrofloxacin was 12.47 µg/ml at 6 hours post IM administration (Fig. 2). The calculated average half-life of enrofloxacin after IM injection is 56 hours. It was not possible to calculate the half-life after oral administration because plasma levels were continuing to increase after 36 hours. Conversion of enrofloxacin to ciprofloxacin was minimal in both trials, never rising above 0.1 µg/ml.
| Figure 1 | 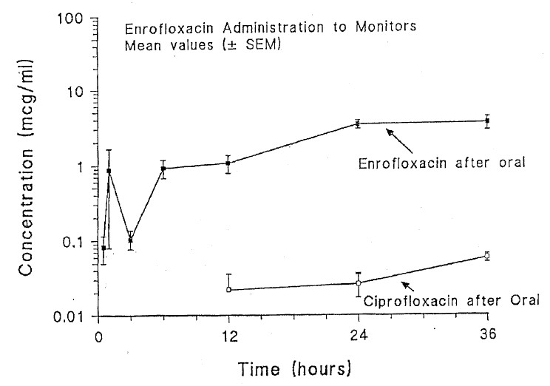
Semilogarithmic plot of plasma enrofloxacin and ciprofloxacin concentrations (µg/ml) versus time in 10 savanna monitors (Varanus exanthematicus) following administration of a single oral dose of enrofloxacin (10 mg/kg). |
|
| |
| Figure 2 | 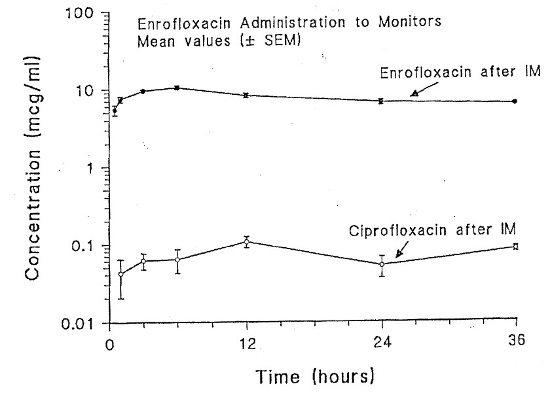
Figure 2. Semilogarithmic plot of plasma enrofloxacin and ciprofloxacin concentrations (µg/ml) versus time in 10 savanna monitors (Varanus exanthematicus) following administration of a single intramuscular dose of enrofloxacin (10 mg/kg). |
|
| |
Discussion
Enrofloxacin has a wide spectrum of antimicrobial activity and a large volume of distribution.7 The minimum inhibitory concentration (MIC) for common reptile pathogens is lower for enrofloxacin then other commonly used antimicrobials, which results in a favorable margin of safety.2 This antibiotic has a broad spectrum of activity which includes the common reptile pathogens: Pseudomonas sp., Aeromonas sp., and Klebsiella sp.4 Pharmacokinetic studies for intramuscularly administered enrofloxacin have been conducted on two species of tortoise (Gopherus polyphemus and Geochelone elegans),5,6 one species of frog (Rana catesbeiana),3 and one species of snake (Python molurus bivittatus).8 No published reports were identified which described the pharmacokinetics of oral enrofloxacin, or any other oral antibiotic, in any reptile species.
Enrofloxacin, and its active metabolite ciprofloxacin, are capable of inhibiting growth of pathogenic bacteria at serum levels of approximately 0.1 µg/ml.3 In savanna monitors, the plasma concentrations exceeded 0.1 µg/ml 30 minutes after intramuscular injection and 6 hours after ingestion. These levels were maintained throughout the sampling period (36 hours) in both trials. For both oral and intramuscular administrations, peak plasma concentrations were two to six times higher than those reported for tortoises, bullfrogs, and pythons.3,5,6,8 Given the long-calculated half-life (56 hours) following intramuscular enrofloxacin, a dosing interval of every 5 days should be considered.
In comparison to plasma concentrations following IM injection, absorption of orally administered enrofloxacin was delayed. Plasma concentrations after 24 hours were similar. Therefore, an initial dose of enrofloxacin (10 mg/kg IM) followed by oral administration for continued therapy would be beneficial for acute infections. In future trials, a lower dosage (5 mg/kg) and longer sampling times up to 100 hours post administration will be evaluated to determine peak levels after oral administration.
Literature Cited
1. Flammer K, Aucoin DP, Whitt DA. Intramuscular and oral disposition of enrofloxacin in African grey parrots following single and multiple doses. J Vet Pharmacol Ther. 1991;14:359–366.
2. Hooper DC, Wolfson JS. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991;324:384–394.
3. Letcher J, Papich M. Pharmacokinetics of intramuscular administration of three antibiotics in bullfrogs (Rana catesbeiana). In: Proceedings of the American Association of Zoo Veterinarians. 1994:79–93.
4. Plumb DC. Enrofloxacin/ciprofloxacin. In: Veterinary Drug Handbook, 2nd ed. Ames, IA: Iowa State University Press; 1995:233–235.
5. Prezant RM, Isaza R, Jacobson ER. Plasma concentrations and disposition kinetics of enrofloxacin in gopher tortoises (Gopherus polyphemus). J Zoo Wild Med. 1994;25:82–87.
6. Raphael BL, Papich M, Cook RA. Pharmacokinetics of enrofloxacin after a single intramuscular injection of Indian star tortoises (Geochelone elegans). J Zoo Wild Med. 1994;25:88–94.
7. Vancutsem PM, Babish JG, Schwark WS. The fluoroquinolone antimicrobials: structure, antimicrobial activity, pharmacokinetics, clinical use in domestic animals, and toxicity. Cornell Vet. 1990;80:173–186.
8. Young LA, Schumacher J, Jacobson ER, Papich M. Pharmacokinetics of enrofloxacin in juvenile Burmese pythons (Python molurus bivittatus). In: Proceedings of the American Association of Zoo Veterinarians. 1994:97.