Abstract
Successful translocation depends on the relocation of healthy wildlife. Medical evaluation, including clinical pathology screening, of animals scheduled for translocation should be conducted.5 Clinical pathology baselines are helpful in evaluating the health status, nutritional state, and the presence of infectious and noninfectious diseases. This study established baseline hematology and biochemistry parameters of eastern North Carolina (USA) North American river otters (Lutra canadensis) live-trapped during the winter months of December–February.
One hundred fifty-five clinically healthy, live-trapped river otters from eastern North Carolina (USA) were sampled over a 4-year period between the dates of December 8 through February 28. Otters were live-trapped, using various-sized standard and modified leg-hold traps, within a 10-county range in central eastern North Carolina for the North Carolina Wildlife Resources Commission (NCWRC) Otter Restoration/Translocation Project (Raleigh, NC, USA). Otters were weighed, then anesthetized using regimens that had been previously published for river otters6,7 or sea otters.8 Complete blood counts were performed using automated cell counters, and 50 sera were submitted for biochemical analysis in 1992 and 1996. Statistical analysis used nonparametric methods because of the non-normal distribution of the data.
Adult male and female clinical pathology values were summarized together, because the statistical differences found were not clinically significant. Microfilaria, most closely resembling Dirofilaria lutrae,4 could be seen by scanning under low magnification (100x) and were found in 18.1% of all blood smears examined during the 4 years of the study. Serum CPK decreased in concentration as the number of days held prior to workup increased. An evaluation of hematology and biochemistry parameters considering degree of injury showed a slight but significant increase in the median leukocyte count (12.9 x103/µl) and absolute numbers of neutrophils (9793.5 µl) as degree of injury increased (Table 1).
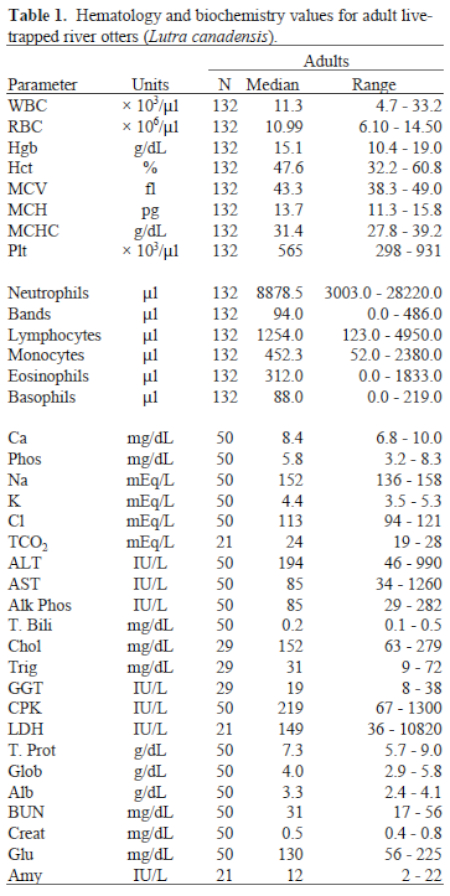
The river otter was found to have a higher concentration of smaller erythrocytes than other species of otters and has a lower blood urea nitrogen, amylase, and creatinine than sea otters (Enhydra lutris).9 There were no significant differences between adult males and females for the study years. It appears that D. lutra is commonly found in river otters in the eastern United States,1-4 including North Carolina. The baseline clinical pathology values established in this study of clinically healthy, live-trapped, wild river otters varied slightly from other otter species, and significant differences were found when comparing age, gender, and trapping injury.
Acknowledgments
This study was funded in part by the North Carolina Zoological Park; the North Carolina Furbearers Association; the College of Veterinary Medicine and the Environmental Medicine Consortium, North Carolina State University.
Literature Cited
1. Davis, H. G., R. J. Aulerich, S. J. Bursian, J. G. Sikarskie, and J. N. Stuht. 1992. Hematologic and blood chemistry values of the northern river otter (Lutra canadensis). Scientifur. 16: 267–271.
2. Hoover, J. P., C. R. Root, and M. A. Zimmer. 1984. Clinical evaluation of American river otters in a reintroduction study. J. Am. Vet. Med. Assoc. 185: 1321–1326.
3. Hoover, J. P., R. J. Baher, M. A. Nieves, R. T. Doyle, M. A. Zimmer, and S. E. Lauzon. 1985. Clinical evaluation and prerelease management of American river otters in the second year of a reintroduction study. J. Am. Vet. Med. Assoc. 187: 1154–1161.
4. Orihel, T. C. 1965. Dirofilaria lutrae sp. N. (Nematoda: Filarioidea) from otters in the southeast United States. J. Parasitol. 51: 409–413.
5. Spalding, M. G. and D. J. Forrester. 1993. Disease monitoring of free-ranging and released wildlife. J. Zoo Wildl. Med. 24: 271–280.
6. Spelman, L. H., P. W. Sumner, J. F. Levine, and M. K. Stoskopf. 1993. Field anesthesia in the North American river otter (Lutra canadensis). J. Zoo Wildl. Med. 24: 19–27.
7. Spelman, L. H., P. W. Sumner, J. F. Levine, and M. K. Stoskopf. 1994. Anesthesia of North American river otters (Lutra canadensis) with medetomidine-ketamine and reversal by atipamezole. J. Zoo Wildl. Med. 25: 214–223.
8. Williams, T. D., F. H. Kocher. 1978. Comparison of anesthetic agents in the sea otter. J. Am. Vet. Med. Assoc. 173: 1127–1130.
9. Williams, T. D., A. H. Rebar, R. F. Teclaw, and P. E. Yoos. 1992. Influence of age, sex, capture technique, and restraint on hematologic measurements and serum chemistries of wild California sea otters. Vet. Clin. Pathol. 21: 106–110.