Abstract
Between November 1993 and March 1997, 19 cases of encephalomyelitis were diagnosed in a closed colony of Japanese macaques (Macaca fuscata). This syndrome had a high fatality rate (84%), commonly affected weaned juveniles, and was characterized by severe lesions of encephalomyelitis mainly involving the white matter. Two of the nine animals treated with antibiotics recovered completely, and passive immunotherapy was beneficial in two cases. Several infectious agents were investigated, but the etiology of this neurological disorder remains undetermined.
Introduction
An outbreak of fatal encephalitis, thought to be associated with a viridans group Streptococcus, has been recently described in a captive colony of Japanese macaques (Macaca fuscata).12 Since this first report, several similar fatalities have occurred in this colony. However, recent findings do not support the initial proposed etiology. The aim of the present communication is to summarize the knowledge regarding this neurological syndrome.
Case Report
Affected animals were members of a group-housed colony of 40 Japanese macaques of the Arashyiama strain used exclusively for behavioral research.3 There had been no new animals nor any major medical problems during the first 10-year period. In November 1992 the colony was moved to an indoor/outdoor facility adjacent to a research barn housing horses, cows, swine, and dogs. The diet consisted of commercial monkey chow, grains, fruit, and vegetables.
Clinical Presentation
Between November 1993 and March 1997, 19 cases (84% mortality rate) were observed in the colony (Table 1). The interval between each case was highly variable (from four to 282 days, mean: 68 days), and cases were usually clustered. With the exception of a seven-year-old animal, all affected animals were juveniles (5–41 months; mean: 22 months). Gender, social rank, and season did not appear to be correlated with disease. Clinical signs were similar among the affected animals, lasting from a few days to over a month. Lethargy, reluctance to move, muscular weakness, and loss of balance were the most common initial clinical signs, which were usually followed by progressive paralysis, head tilt, and hemifacial paresis. Attempted treatments and clinical outcome are presented in Table 2. Two of the nine animals treated with antibiotics recovered completely. A third animal with advanced disease (lateral recumbency) recovered following intravenous administration of mixed sera from the two previous survivors. Another animal improved after intravenous injection of mixed sera, but subsequently deteriorated and was euthanatized.
Table 1
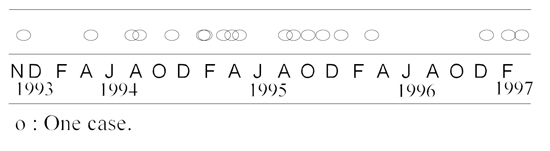
Encephalomyelitis in 19 Japanese macaques. Distribution of the cases (November 1993 to March 1997)
Table 2. Treatments and outcomes in Japanese macaques with encephalomyelitis. No treatments were attempted on animals 1, 2, 11, 12, and 14
|
No.
|
Treatment
|
Outcome
|
|
3
|
Oxytetracyclinea (25 mg/kg IM, BID, 10 days)
|
Euthanatized
|
|
4
|
Enrofloxacinb (5 mg/kg, IM, BID, 14 days)
|
Recovered
|
|
5
|
Enrofloxacin (5 mg/kg, IM, BID, 5 days)
dexamethasonec (0.17 mg/kg IM, SID, 4 days)
|
Euthanatized
|
|
6
|
Enrofloxacin (5 mg/kg, IM, BID, 8 days)
penicillin Gd (20000 IU/kg, IM, BID, 8 days)
dexamethasone (0.2 mg/kg IM, SID, 2 days)
|
Euthanatized
|
|
7
|
Gentamicine (1.5 mg/kg IM, TID, 11 days)
ampicillinf (83 mg/kg SC, TID, 11 days)
|
Recovered
|
|
8
|
Gentamicin (1mg/kg IM, SID, 8 days)
ampicillin (83 mg/kg SC, BID, 8 days)
enrofloxacin (15 mg/kg, IM, BID, 6 days)
dexamethasone (1.7 mg/kg IM, BID, 6 days)
|
Euthanatized
|
|
9
|
Enrofloxacin (5 mg/kg, IM, BID, 8 days)
dexamethasone (1.25 mg/kg IM, BID, 3 days)
|
Euthanatized
|
|
10
|
Gentamicin (2.6 mg/kg IM, BID, 4 days)
ampicillin (70 mg/kg SC, BID, 8 days)
dexamethasone (1.4 mg/kg IM, BID, 4 days)
|
Euthanatized
|
|
13
|
Enrofloxacin (5 mg/kg, IM, BID, 8 days)
|
Dead
|
|
15
|
Serum from animals Nos. 7 and 4 (18+9 ml)
|
Recovered
|
|
16
|
Serum from animals Nos. 7, 4, and 15 (10 ml)
|
Improved, then relapsed,
euthanatized
|
aLiquamycin-LP® (Rogar/STB Inc., Pointe-Claire, QC)
bBaytril® (Bayvet, Concord, ON)
cCentrazone-5® (Central Sales LTD., Brampton, ON)
dEthacillin® (Rogar/STB Inc., Pointe-Claire, QC)
eGentocin® (Schering Canada Inc., Pointe-Claire, QC)
fAmpicillin sodium® (Novopharm, Toronto, ON)
Pathology
A complete postmortem examination, as described elsewhere,12 was performed on 16 animals. Gross lesions were similar in all animals and consisted of circular yellow areas 2–7 mm in diameter; sprinkled with hemorrhages; located within the white matter of the brain, the cerebellar medulla, and the spinal cord. Microscopically, most cerebral lesions were located at the junction of the grey and white matter and were characterized by well-demarcated areas of severe neuropile vacuolation with moderate to severe hemorrhages. Necrotic leukocytoclastic vasculitis, characterized by segmental fibrinoid necrosis of the media and severe infiltration of the vascular walls by a dense population of neutrophils, was observed in several cases. Moderate numbers of neutrophils were scattered in the leukomalacia foci and were more numerous in areas adjacent to vessels. Perivascular lymphoid cuffs and mild diffuse gliosis were present in the cerebral tissue surrounding the lesions. Infiltration of leukomalacia foci by gitter cells was predominant in animals that survived for a longer period. Microorganisms were not observed in these lesions using Gram and periodic acid-Schiff stains. Screening for equine, bovine, canine, and feline herpesviruses, Listeria monocytogenes, Haemophilus somnus, and Toxoplasma gondii was negative on formalin-fixed brain from two animals using an immunofluorescence technic (Dr. Fabio del Piero, College of Veterinary Medicine, Cornell University, Ithaca, NY). A few colonies of viridans group Streptococcus, similar to S. salivarius, were isolated from a cerebral lesion of the first three animals, and from the lungs of the fourth case. This bacterium was not found in any of the subsequent cases, and microagglutination tests against the same Streptococcus failed to demonstrate the presence of significant titers in the sera of six affected animals. Viruses or bacteria were not detected during electron microscopy examination of glutaraldehyde-fixed cerebral tissues from four animals. Viral isolation from frozen brain and cerebellum of two cases yielded negative results (see Hilliard et al.10 for methodology).
Serology
The following 13 agents were confirmed absent from the colony: Coxiella burnetii (0 positive/10 sera tested); Leptospira sp., 8 serovars (0/10); Borrelia burgdorferi (0/20); Western equine encephalitis (0/20); Eastern equine encephalitis (0/20); Powassan equine encephalitis (0/20); St. Louis encephalitis (0/20); hantavirus (0/20); poliovirus (0/32); Herpesvirus simplex-1 and 2 (0/32); Varicella zoster virus (0/32); simian immunodeficiency virus (0/4); and simian retrovirus (0/4). Anti-Herpesvirus simiae (B virus) antibodies were found in 20 out of 32 sera tested, but in none of the three survivors. All 32 sera tested for the presence of anti-Epstein Barr virus were positive. However, seroconversion was not observed in the three survivors. Antibodies against cytomegalovirus were detected in 13 of the 32 sera tested but were not present in two of the three survivors, and seroconversion was not observed in the third animal. Simian T-cell leukemia virus-antibodies were present in one of the four sera tested. Finally, 50% of the sera tested (16/32) were positive for measles virus. However, four of the symptomatic macaques were seronegative, and seropositive clinical cases had either similar or smaller titers that asymptomatic animals.
Discussion
Despite extensive investigations, the etiology of the neurological disorder endemic to this colony could not be determined. Neurological diseases are not uncommon in primates of the genus Macaca, and have been associated with different bacterial,2,4,6-8 viral,1,5,16 and parasitic14 pathogens. In Japanese macaques, reported cases of neurological disorders include a case of canine distemper virus infection,17 and possible coyotillo berry intoxication.11 Clinical and pathological findings observed in our colony differ from all conditions reported previously in macaques.
The epidemiological pattern of this condition (clustering of cases; predisposition for immature, weaned animals) and the benefit of passive immunotherapy favor an infectious etiology. Based on the isolation of the same bacterial species in the first four animals and on the successful treatment of two cases with antibiotics, a bacterial etiology was previously proposed for this syndrome.12 The failure to isolate this microorganism from the 11 subsequent cases and the ineffectiveness of the antimicrobial treatment on seven affected animals make this hypothesis unlikely.
Histologic lesions observed in the brains and spinal cords shared some similarities with those described in acute disseminated encephalomyelitis (ADEM) in humans,15 and in experimental autoimmune encephalomyelitis.13 ADEM is believed to be associated with an acute autoimmune reaction to myelin, and is usually triggered by different viral infections, such as measles, infectious mononucleosis (Epstein-Barr virus), influenza, parainfluenza, and mumps. Both Epstein-Barr and measles viruses were prevalent in our colony. However, the absence of titers and/or seroconversion in several of the affected animals does not support their implication in this syndrome. A search for the presence of antibodies against mumps, influenza, and parainfluenza viruses could not be performed. In addition, the success of the passive immunotherapy and the failure of corticosteroid therapy make diagnosis of postinfectious encephalomyelitis unlikely. Finally, it should be realized that ADEM is extremely rare in humans. An epidemic pattern as observed in this colony would be highly unusual for an autoimmune disorder.
Animals from this group have been exposed to simian T-cell leukemia virus. This virus has been previously reported in free-ranging Japanese macaques,9 but has never been associated with neurological problems. In addition, no titers were found in three of the four affected animals tested. Despite their prevalence, Herpesvirus simiae (B virus) and cytomegalovirus do not appear to be involved in this condition since seroconversion or titers for these viruses were not observed in several of the affected animals. Finally, the negative serology to the immunosuppressive viruses (simian immunodeficiency virus and simian retrovirus), combined with no previous evidence of these viruses in the colony, makes it highly unlikely they played a role in this disease.
Additional investigations for other potential etiologies, such as spumavirus, retrovirus, and non-B herpesviruses, are still in progress.
Acknowledgments
We are grateful to Drs. Guy Fitzgeral, Robert Higgins, Daniel Martineau, Khyali Mittal, and André Ravel (Université de Montréal); to Drs. Radmila Mirkovic and Julia Hilliard (Southwest Foundation for Biomedical Research); and to Dr. Timothy O’Neil (Registry of Comparative Pathology, AFIP) for useful discussion. We also thank Dr. Fabio del Piero (College of Veterinary Medicine, Cornell) for the immunochemistry work; Drs. Denise Deniscourt and Harvey Artsob (Health Canada) for the serology; and Paul Vasey, Dr. Jean Prud’homme, and Dr. Guy Fitzgeral (Université de Montréal) for technical assistance.
Literature Cited
1. Anderson DC, Swenson RB, Orkin JL, Kalter SS, McClure HM. Primary Herpesvirus simiae (B-Virus) infection in infant macaques. Lab Anim Sci. 1994;44:526–530.
2. Chalifoux LV, Hajema EM. Septicemia and meningoencephalitis caused by Listeria monocytogenes in a neonatal Macaca fascicularis. J Med Primatol. 1981;10:336–339.
3. Chapais B, Prud’homme J, Teijeiro S. Dominance competition among siblings in Japanese macaques: constraints on nepotism. Anim Behav. 1994;48:1–13.
4. Cobb LM, Hepworth PL, Heywood R. Pneumococcal leptomeningitis in cynomolgus monkeys (Macaca fascicularis). Vet Rec. 1976;99:84.
5. Daniel MD, Garcia FG, Melendez LV, Hunt RD, O’Connor J, Silva D. Multiple Herpesvirus simiae isolation from a rhesus monkey which died of cerebral infarction. Lab Anim Sci. 1975. 25:303-308.
6. Fox JG, Rohovsky MW. Meningitis caused by Klebsiella spp. in two rhesus monkeys. JAVMA. 1975;167:634–636.
7. Gilbert SG, Reuhl KR, Wong JH, Rice DC. Fatal pneumococcal meningitis in a colony-born monkey (Macaca fascicularis). J Med Primatol. 1987;16:333–338.
8. Graczyk TK, Cranfield MR, Kempske SE, Eckhaus MA. Fulminant Streptococcus pneumoniae meningitis in a lion-tailed macaque (Macaca silenus) without detected signs. J Wild Dis. 1995;31:75–78.
9. Hayami M, Komuro A, Nozawa K, Shotake T, Ishikawa K, Yamamoto K, Ishida T, Honjo S, Hinuma Y. Prevalence of antibody to adult T-cell leukemia virus-associated antigens (ALTA) in Japanese monkeys and other non-human primates. Int J Cancer. 1984;33:179–183.
10. Hilliard JK, Munoz RM, Lipper SL, Eberle R. Rapid identification of Herpesvirus simiae (B virus) DNA from clinical isolates in nonhuman primate colonies. J Virol Methods. 1986;13:55–62.
11. Joiner GN, Russell LH, Bush DE, Gleiser CA, Johnston TD, Fedigan L. A spontaneous neuropathy of free-ranging Japanese macaques. Lab Anim Sci. 1975;25:232–237.
12. Lair S, Chapais B, Higgins R, Mirkovic R, Martineau D. Myeloencephalitis associated with a viridans group Streptococcus in a colony of Japanese macaques (Macaca fuscata). Vet Pathol. 1996;33:99–103.
13. Lerner EM 2nd, Stone SH, Myers RE. Autoimmune encephalomyelitis and hemorrhagic retinal disease in neonatal, infant, juvenile, and adult monkeys. J Neuroimmunol. 1985;7:299–313.
14. Olson LC, Skinner SF, Palotay JL, McGhee GE. Encephalitis associated with Trypanosoma cruzi in a Celebes black macaque. Lab Anim Sci. 1986;36:667–670.
15. Sriram S, Steinman L. Postinfectious and postvaccinal encephalomyelitis. Neurol Clin. 1984;2:341–353.
16. Steele MD, Giddens WE Jr, Valerio M, Sumi SM, Stetzer ER. Spontaneous paramyxoviral encephalitis in nonhuman primates (Macaca mulatta and M. nemestrina). Vet Pathol. 1982;19:132–139.
17. Yoshikawa Y, Ochikubo F, Matsubara Y, Tsuruoka H, Ishii M, Shirota K, Nomura Y, Sugiyama M, Yamanouchi K. Natural infection with canine distemper virus in a Japanese monkey (Macaca fuscata). Vet Microbiol. 1989;20:193–205.