Role of Chronic Iron Overload in Multiple Disorders of Captive Black Rhinoceroses (Diceros bicornis)
Abstract
Necropsy reports of black rhinoceroses (Diceros bicornis) dying in captivity have frequently cited hemosiderosis as residual evidence of hemolytic anemia, a disorder of high morbidity and mortality in this species. Recent necropsy experience, however, and reevaluation of archival materials, revealed histopathologic patterns and quantities of iron deposition that were incompatible with that interpretation. Extensive, sometimes massive, involvement of both reticuloendothelial and parenchymal cells in multiple organs, indicated that this condition represents a true iron overload syndrome and could not result from hemolytic disease alone. This was confirmed by measurements of iron analytes in fresh and stored sera from four species of rhinoceroses, and by quantitative analyses of tissue iron from a limited number of archival specimens. Comparisons with free-ranging rhinoceroses indicated that iron accumulates as a consequence of captive conditions and in direct relation to time in captivity, producing an acquired hemochromatosis. Histopathologic characteristics of iron deposition in affected rhinoceroses were virtually identical to those observed in humans and lemurs with dietary iron overload syndromes. No evidence of iron excess was found in natural grazers (Ceratotherium simum and Rhinoceros unicornis), but sera from three Sumatran rhinoceroses (Dicerorhinus sumatrensis) revealed comparable elevations, indicating that species that normally forage predominantly on browse are most at risk for development of hemochromatosis in captivity. Since enteric absorption was the only possible source of iron excess, these observations suggest that components that bind iron into nonabsorbable complexes (such as tannins, phytates, polyphenolics, etc.), present in dietary browse and/or absent in captive herbivore diets, might be responsible for increased bioavailability of iron and its unregulated uptake. Since free iron induces cell injury by catalyzing production of highly reactive hydroxyl free radicals, to which rhinoceros red cells are highly sensitive, iron overload might contribute directly to cellular impairment in any of the several syndromes affecting black rhinoceroses in captivity. In particular, the suppressive effect of iron excess on “nutritional immunity” (host vs. pathogen competition for essential elements) is likely to contribute to this species’ apparently high susceptibility to a broad range of infectious diseases. Strategies to prevent and/or treat iron overload in captive rhinoceroses may be restricted to the juvenile population, because of the inordinate body burdens present in many older animals and practical limitations on the amounts of iron that can be mobilized by phlebotomy and/or chelation therapy.
Introduction
Necropsy records of captive black rhinoceroses (Diceros bicornis) dying over the past three decades have commonly mentioned the presence of hemosiderosis, inordinate tissue deposition of the iron storage pigment hemosiderin. This has generally been viewed as an incidental finding and interpreted as evidence of previous hemolytic episodes in which premature destruction of red cells allows hemoglobin iron to accumulate in reticuloendothelial cells throughout the body. The magnitude and patterns of iron deposition that we have observed in more recent necropsies, however, have been incompatible with that interpretation, conforming instead to an acquired form of hemochromatosis affecting parenchymal as well as reticuloendothelial cells, thereby producing a pathologic iron-overload syndrome with potentially injurious effects on multiple organ systems.8 Assessment of this condition by assays of serum iron compounds has now confirmed that captive black rhinoceroses exhibit significant increases in total body iron stores, with serum ferritin concentrations often tenfold to many hundreds of times higher than those in their natural habitats. Assays on a limited number of Sumatran rhinoceroses (Dicerorhinus sumatrensis) revealed that they too, are overloaded with iron in captivity.
Hemosiderosis is but one of a number of disorders or syndromes of unknown etiology that occur frequently in captive black rhinoceroses, often with severe to lethal consequences. These include episodic hemolytic anemia; high susceptibility to Leptospira, Salmonella, mycobacteria, and fungal pneumonias; diffuse ulcerations of skin and mucous membranes; congenital leuko-encephalomalacia; hepatic failure; chronic progressive anemia with severe weight loss and occult infection; and a more recently recognized syndrome involving the microvasculature, idiopathic hemorrhagic vasculopathy. That so many disparate disorders should affect only one species of rhinoceros while sparing others seems intuitively improbable, unless they share some common elements in terms of underlying causes or pathogenesis.11 Data presented here suggest that iron overload might serve as a common mediator, potentially contributing to the initiation, clinical course, and severity of a number of these disease syndromes.
Methods
Over the past several years, the zoological community has accorded the senior author opportunities to attend and assist in necropsies of black rhinoceroses dying from (or euthanatized for) diverse problems, including traumatic, infectious, hematologic, and hemorrhagic disorders. Histopathologic materials from past necropsies were also made available by a number of institutions for review and reevaluation, including recuts of paraffin blocks and special stains.
Blood specimens from captive and free-ranging black rhinoceroses (and other species) that were referred to the UCLA Hematology Research Laboratory for evaluation of blood cell metabolism were analyzed additionally for serum iron compounds. Archival specimens stored at individual institutions, including the collective repository for black rhinoceroses at the St. Louis Zoo, were also sought, obtained, and similarly studied. Serum iron concentrations, iron binding capacities, and transferrin saturations were determined using the quantitative colorimetric technique employed in Sigma diagnostics (St. Louis, MO) Kit No. 565. Serum concentrations of ferritin and haptoglobin and elemental iron assays of frozen necropsy tissues were contracted to the Cellular and Molecular Pathology Laboratory at Kansas State University College of Veterinary Medicine where species-specific iron-analyte assays for rhinoceroses were originally developed by the late Dr. Joseph Smith and his associates.12
Results
Necropsy Evidence of Iron Overload
Iron stores in necropsy tissues were significantly (sometimes massively) increased in virtually all captive adult black rhinoceroses studied, regardless of (and out of proportion to) any previous or current hemolytic process. All but three had negative histories for past hemolytic episodes, and normal haptoglobin and bilirubin values ruled out active hemolysis. Hemosiderin deposition appeared far too large in amount, and its distribution in multiple organs too atypical and widespread, to be caused either by reprocessing of normally aged red cells or by their premature destruction. Erythroid hyperplasia and/or extramedullary erythropoiesis, and renal tubular epithelial siderosis characteristic of intravascular hemolysis, were uniformly absent. Bone marrows tended to be hypocellular and even fibrotic with broad sheets of hemosiderin-laden macrophages filling medullary spaces, producing in some instances a myelophthisic siderosis.
Histopathologic characteristics of pigment deposition were virtually identical to dietary iron-overload syndromes occurring in other captive wildlife such as lemurs13 and in sub-Saharan African Bantu tribes whose methods of food and beverage preparation significantly enhance bioavailability of ingested iron.2,3 (In the latter population, as in human idiopathic hemochromatosis, genetic determinants also contribute to increased uptake of dietary iron.4) Organs most consistently involved were spleen, liver, bone marrow, and lungs, with less frequent but prominent siderosis of intestines, lymph nodes, heart, adrenals, thyroid, and other endocrine organs. Distinctive deposits were present in the lamina propria and submucosa of the entire bowel with the highest concentrations occurring at the tips of small bowel villi, a histologic pattern indicative of enteric origin of excess iron.6 Deposition within macrophages in these organs generally dominated, but parenchymal cells were also frequently involved to an extent that would be expected to affect cellular function.
Dense patterns of hepatic iron deposition observed in these cases differed distinctly from those described in uncomplicated hemolytic disease. Portal macrophages and sinusoidal Kupffer cells were heavily engorged with hemosiderin. Hepatocytes contained course and fine iron-positive granules that were most prominent in periportal regions, but extended throughout the lobules. Despite extensive liver deposits in many cases, there was minimal evidence of hepatic fibrosis that characteristically occurs in severe hemochromatosis in humans and other species, but cirrhotic changes and low-grade hepatocellular carcinoma have been observed in archival material from one black rhinoceros.
Serum Iron Analytes in Captive and Free-Ranging Rhinoceroses
These subjective impressions of inexplicably severe hemosiderosis at necropsy were supplemented by quantitative assays of serum iron and ferritin concentrations and iron-binding capacity in seven groups of rhinoceroses (Table 1). Since the six animals in Group D had lived lifelong in the wild until capture approximately 2 wk before sera were obtained, they provided the best control standard for comparative purposes. This was the only group of black rhinoceroses studied in which iron analyte values for all members fell uniformly within established normal ranges for humans and equines.
Table 1. Serum iron assays in various species of captive and free-ranging rhinoceroses
|
Group
|
Species and location
|
Time in captivity prior to sampling
|
Iron (µg/dl)
|
Transferrin saturation (%)
|
Ferritin (ng/ml)
|
|
A
|
D. bicornis, long-term U.S. captivity
|
x=144 mo
(1–391 mo)
|
247±94 (34)*
|
65±22
|
2,200±2,240
|
|
B
|
Wild D. bicornis imported Zimbabwe to U.S., 1992
|
~10 mo
|
243±103 (9)
|
72±20
|
1,270±566
|
|
C
|
D. bicornis in Zimbabwe boma confinement
|
x=86 mo
(12–150 mo)
|
144±44 (6)
|
44±13
|
372±160
|
|
D
|
D. bicornis lifelong free-ranging until capture
|
<2 wk
|
101±19 (6)
|
28±6
|
133±62
|
|
E
|
C. simum, U.S. captivity
|
Long-term
|
134±43 (6)
|
37±15
|
47±32
|
|
F
|
R. unicornis, U.S. captivity
|
Long-term
|
123±48 (4)
|
33±8
|
15±4
|
|
G
|
D. sumatrensis, U.S. captivity
|
Long-term
|
222±65 (3)
|
91±3
|
798±134
|
|
Equine controls
|
50–198
|
22–44
|
43–261
|
|
Human controls
|
65–165
|
20–50
|
10–300
|
*Values shown are x±1 SD. Number of animals assayed is shown in parentheses.
Table excludes values from long-term U.S. captive black rhinoceroses with serum ferritin concentrations >10,000 ng/ml (see Fig. 1).
Figure 1. Serum ferritin concentrations in various groups of rhinoceroses (log scale)
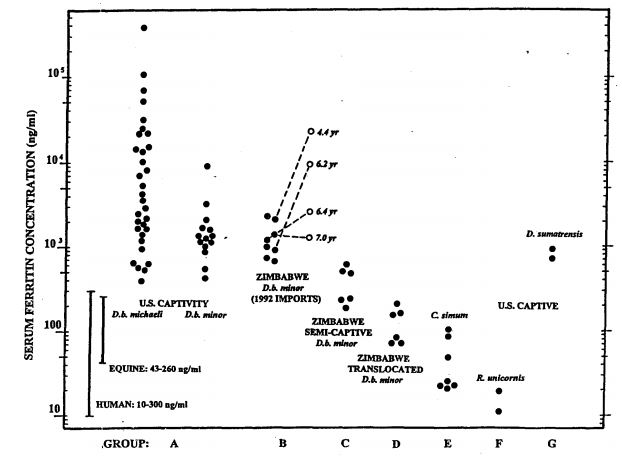
Eleven of the twelve highest values in group A (>10,000 ng/ml) occurred in kindreds of calves with congenital leukoencephalomalacia. This group also had high incidences of primary hemolytic anemia and mucocutaneous ulcerative disease. Open symbols in Group B are values derived from follow-up samples obtained after residing in U.S. captivity for the periods indicated.
Average serum iron among long-term U.S. captives (Group A) and recent imports (Group B) was 2½ times higher than the mean for control Group D, and individual values ranged as high as fivefold or greater. Mean transferrin saturation in these long-term captives was also more than twice as high as Group D control mean. Group C, with much shorter periods in captivity, displayed correspondingly intermediate values for serum iron concentrations with mean transferrin saturations of 44%. Smith et al.12 using other assay techniques, reported even higher serum iron (x = 614±200 mg/dl) with similar transferrin saturations (x = 67.5±3.7 %) for long-term captive Diceros. In human hemochromatosis, transferrin saturations of 65–70% are viewed as the threshold region for onset of overt organ damage by chronic iron overload and its sequelae.2
Within certain ranges, serum ferritin concentrations provide the best estimates of total body iron stores short of quantitative assays of hepatic and other tissues. Results shown in Fig. 1 demonstrate concentrations in captive black rhinoceroses that ranged fully over three orders of magnitude, dramatically greater than other species of rhinoceroses and various control groups. Two neonatal samples from calves born in captivity had <200 ng/ml ferritin, but ferritin concentrations tended to increase logarithmically thereafter as a function of time in captivity (data not shown), confirming similar observations made previously by Kock et al.5 and Smith et al.12 The presence of significant increases in total body burdens of iron, demonstrated by necropsy pathology and serum iron analyte assays, received additional confirmation from quantitative assays of stored frozen tissues from selected animals (data not shown). It is particularly notable that iron analytes in predominantly grazing rhinoceroses (white and Indian) showed no elevations in captivity, but the other browsing species (Sumatran) distinctly did, reaching levels comparable to some long-term captive Diceros.
Discussion
The significance of hemosiderosis in captive black rhinoceroses was brought into sharper focus by two pivotal studies. In 1993, Kock and her associates reported that hemosiderosis was not seen in necropsies of free-ranging Diceros in Zimbabwe that died within 2 wk of capture, whereas it began to appear and progressively increase in those that died during boma confinement >2 wk to 2 yr after capture.5 Similarly, Smith et al. found that serum ferritin levels in U.S. captive black rhinoceroses tended to increase progressively with age or time in captivity, and further, that ferritin and hepatic iron stores were both significantly higher in captive black vs. white rhinoceroses.12 Since haptoglobin concentrations were comparable between the two species, hemolysis could not account for these discrepancies, and the authors presciently suggested that dietary changes resulting in increased iron absorption might provide a more logical explanation.
Taken together, these two important studies present perhaps the most significant differences yet reported between captive black and white rhinoceroses and between Diceros in captivity and those residing lifelong in the wild. A pertinent aspect of both studies was an apparent correlation between time in captivity and degree of iron overload. Supplemented by our own more recent necropsy observations and quantitative laboratory assessments of body iron stores reported here, we believe that these data present compelling evidence for existence of a clinically significant iron overload syndrome in black rhinoceroses that is directly related to captive conditions, most likely on a dietary basis. The possibility that enhanced uptake of iron in these animals might also be influenced by genetic determinants, such as those operative in human hemochromatoses,2-4 remains to be investigated.
Hemosiderosis has also been a common finding in other species of animals when brought into captivity, including simians, prosimians, and avians, whose natural diets (like the browsing rhinoceroses) normally contain high concentrations of tannins, phytates, fiber, polyphenolics, phosphates, and other compounds that chelate iron into insoluble complexes, most of which then passes through the gastrointestinal tract unabsorbed. Both Sumatran and black rhinoceroses normally browse on trees and shrubs. By contrast, white (Ceratotherium simum) and Asian greater one-horned (Rhinoceros unicornis) rhinoceroses predominantly graze on grasses, and neither shows biochemical or necropsy evidence of iron overload in captivity. In the case of browsing rhinoceroses, even though the content of iron may be the same in natural-browse vs. captive diets, we believe that the absence of natural chelators in grass-based preparations likely increases the bioavailability of iron, allowing it to accumulate progressively in animals sustained largely on captive rations.
Although speculative, a number of potential connections exist between iron overload and certain disorders affecting these species in captivity. It is well established that susceptibility to infections, for example, is significantly increased in iron-loaded humans and other animals. Since microorganisms compete with their hosts for certain metabolites, a major defense mechanism (so-called “nutritional immunity”) involves rapid sequestration of iron to deprive invading microorganisms of ready access to this essential trace element.14 Most microorganisms thrive in high-iron environments, both in vitro and in vivo, and many (such as mycobacteria) are notoriously virulent in humans and animals with iron overloads. This presents a major clinical problem for children with thalassemia or other hemolytic disorders who develop iron overloads from frequent transfusions, and it directly underlies the high incidence and virulence of tuberculosis currently rampant among hyperferremic African Bantu.7
Another possible connection exists between iron overload and the myelin degenerative changes occurring in congenital leukoencephalomalacia. Among U.S. captive black rhinoceroses, (see Fig. 1), 11 of the 12 highest serum ferritin concentrations were found in immediate relatives of the four female calves known to have died with this disorder. In each instance, their mothers had extraordinary elevations (hundredfold to thousandfold greater than normal), and transplacental transfer (pronounced, but transient, neonatal hyperferritinemia) was clearly demonstrable in the female calves, but, for unknown reasons, not in their male siblings.9
Free iron produces cell injury primarily by production of hydroxyl free radicals, to which rhinoceroses are known to be highly vulnerable,10 a problem that may be compounded by inherently low levels of the antioxidant, vitamin E. Free radicals actively attack lipid membrane components of cells and organelles, alter critical structural and enzymatic proteins, and cleave DNA, disrupting cellular replication. The consequences of such events during in utero development would likely be disastrous, and might even be related to the male preponderance and survival rates among live births in North America.1
Whether or not iron overload is causally related to any of the diverse disorders affecting captive black rhinoceroses, these findings indicate that iron burdens have reached hazardous proportions in this captive population and (based on a small sample) in Sumatran rhinoceroses as well. Toxic effects of iron overload in humans and other mammals occur at levels far lower than those now prevalent in these two species. Were human clinical criteria to be applied, virtually all captive browsing rhinoceroses that we have been able to evaluate would be immediate candidates for therapeutic intervention by phlebotomy or parenteral chelation.
The potential hazards of progressive body-iron burdens in browsing rhinoceroses merit consideration of both preventative and therapeutic strategies. Addition of high-tannin food components, such as tamarind pods, is already being tried by some zoo veterinary staffs, and mobilization and excretion of tissue iron has been demonstrated in one black rhinoceros by chelation therapy with desferrioxamine preparations. Therapeutic intervention, as well as preventative measures, might require a triage approach, targeting the youngest segment of the captive population with the lowest body iron burdens and the greatest breeding potential. Calves are apparently born with low body iron stores, but ferritin levels may rise tenfold by 3–4 yr of age. The older the animal, or the longer its time in captivity, the less effective intervention is likely be, since their progressively larger storage pools of iron would be relatively less affected by modulation of daily intake or significantly altered by any practical means of iron mobilization within their lifetimes.
For animals trained to tolerate the procedure, phlebotomy has a number of potential advantages: It is relatively non-intrusive and free of side effects, inexpensive, and it allows precise calculation and monitoring of quantities of iron removed, since hemoglobin contains a fixed amount of iron (0.34% by weight). Phlebotomy also provides an opportunity for secondary benefits: harvested plasma could easily be preserved, and by slightly more complicated procedures, washed red cells could be frozen in glycerol and stored long-term for potential transfusional use.
Because virtually nothing is yet known about the dynamics of iron homeostasis in these species, carefully planned and controlled studies are needed before widespread application of any preventative or therapeutic approach can be recommended. In the interim, it would seem prudent to assess trace- and transition-metal status in all captive black and Sumatran rhinoceroses, particularly those active in breeding programs.
Acknowledgments
We are particularly grateful to the many individuals and institutions in the zoo and wildlife community who invited the senior author to participate in necropsies over the past 7 yr, and to those who shared valuable archival sera and frozen tissues, and who provided important histopathology materials for reevaluation. Serum ferritin, haptoglobin, and tissue iron assays performed by Sue Chavey in the Cellular and Molecular Pathology Laboratory at the University of Kansas College of Veterinary Medicine, under the direction of Dr. Gordon A. Andrews, were essential for quantitative confirmation of subjective necropsy observations. Angel Tsu provided the technologic expertise for iron, enzyme, and metabolite assays performed in the UCLA Hematology Research Laboratory. Research support was provided by grants from the International Rhino Foundation, the Morris Animal Foundation, the LB Research and Education Foundation, and a Fulbright Senior Research Scholar award from the J. William Fulbright Foreign Scholarship Board.
These studies are dedicated to the memory of Dr. Joseph E. Smith, friend and colleague to so many of us in both veterinary and human medicine, whose tragic untimely passing prevented him from completing his seminal work in this field.
Literature Cited
1. Atkinson, S.J., E.S. Dierenfeld, and T.J. Foose. 1997. Possible determinants of skewed natal sex ratios in captive black (Diceros bicornis) and Indian (Rhinoceros unicornis) rhinoceros in North America. Report to the International Rhino Foundation.
2. Bothwell, T.H., R.W. Charlton, and A.G. Motulsky. 1995. Hemochromatosis. Chapter 69 in C.R. Scriver, A.L. Beaudet, W.S. Sly, D. Valle (eds.) The Metabolic and Molecular Bases of Inherited Disease, 7th ed. New York: McGraw-Hill. 2237–2269.
3. Gordeuk, V.R. 1992. Hereditary and nutritional iron overload. Bailliere’s Clin. Haematol. 5:169–186.
4. Gordeuk, V.R., J. Mukiibi, S.J. Hasstedt, W. Samowitz, C.Q. Edwards, G. West, S. Ndambire, J. Emmanual, N. Nkanza, Z. Chapanduka, M. Randall, P. Boone, P. Romano, R.W. Martell, T. Yamashita, P. Effler, and G. Brittenham. 1992. Iron overload in Africa. Interaction between a gene and dietary iron content. New Engl J Med 326:95–100.
5. Kock, N., C. Foggin, M.D. Kock, and R. Kock. 1992. Hemosiderosis in the black rhinoceros (Diceros bicornis): A comparison of free-ranging and recently captured with translocated and captive animals. J. Zoo Wildl. Med. 23:230–234.
6. Lee, G.R., S. Nacht, J.N. Lukens, and G.E. Cartwright. 1968. Iron metabolism in copper-deficient swine. J. Clin. Invest. 47:2058–2069.
7. Moyo, V.M., I.T. Gangaidzo, V.R. Gordeuk, C.F. Kiire, and A.M. MacPhail. 1997. Tuberculosis and iron overload in Africa: a review. Central Afr. J. Med. 43:334–339.
8. Paglia, D.E., and P. Dennis. 1999. Acquired hemochromatosis in captive black rhinoceroses. (In preparation for Nature)
9. Paglia, D.E., D.E. Kenny, E.S. Dierenfeld, and I-H. Tsu. 1999. Potential role for iron overload in the pathogenesis of leukoencephalomalacia in captive black rhinoceroses (Diceros bicornis). (In preparation for J Zoo Wildl Med)
10. Paglia, D.E., and R.E. Miller. 1993. Erythrocytes of the black rhinoceros (Diceros bicornis): susceptibility to oxidant-induced haemolysis. Intl. Zoo Yearbk. 32:20–27.
11. Paglia, D.E., R.E. Miller, and S.W. Renner. 1996. Is impairment of oxidant neutralization the common denominator among diverse diseases of black rhinoceroses? Proceedings of the AAZV, Puerta Vallarta, Mexico. 37–41.
12. Smith, J.E., P.S. Chavey, and R.E. Miller. 1995. Iron metabolism in captive black (Diceros bicornis) and white (Ceratotherium simum) rhinoceroses. J. Zoo Wildl. Med. 26:525–531.
13. Spelman, L.H., K.G. Osborn, and M.P. Anderson. 1989. Pathogenesis of hemosiderosis in lemurs: Role of dietary iron, tannin, and ascorbic acid. Zoo Biol. 8:239–251.
14. Weinberg, E.D. 1978. Iron and infection. Microbiol. Rev. 42:45–66.