Abstract
Tilapia (Oreochromis niloticus) are a valuable and widely used fish, both as a food source and as a biologic model in the scientific community.2 Culture of tilapia is a lucrative business world-wide, with farms producing over 1,000,000 pounds of fish annually. Problems of infection with bacterial pathogens like Vibrio spp. and Streptococcus spp.4 often wipe out tilapia farms as well as spread infection to the human caretakers. Because of the growing concern about antibiotic residues in food for human consumption, as well as the increase in antibiotic resistant microorganisms, vaccination has gained notoriety as a safe, inexpensive, and effective means to protect animals from infectious diseases. DNA vaccination is the most recent addition to the arsenal of vaccines. It consists of plasmid DNA containing sequences encoding a protein of a pathogen. This plasmid is taken up by host cells, where the encoded proteins are made and presented, initiating an immune response. Nucleic acid vaccines have elicited protective immune responses against a variety of pathogens, including bacteria1 and parasites,3 in many different vertebrate species. In order to evaluate the humoral immune response in tilapia to such an inoculation, a DNA vaccine was produced using a bacterial β-galactosidase reporter gene expression plasmid. The reporter gene system allows expression of a protein, that is foreign to the host but is non-pathogenic, which initiates an immune response. In the study, thirty purebred O. niloticus were first grouped according to weight, so a similar number of fish at the same weight range were in each group. Then the fish were randomly allocated into three groups of ten fish each. Group 1 fish each received 50 µg of the test plasmid, pCMVβ, containing the β-galactosidase gene. Group 2 fish each were immunized with 50 µg of the empty, control plasmid, pCI, that lacks the reporter gene. Group 3 fish each received 100 µl sterile saline as a diluent control. Each treatment was administered by intramuscular injection. Blood was collected prior to the immunization and then every other week for a total of five blood samples for each fish. The samples were screened for β-galactosidase antibodies using indirect enzyme-linked immunosorbent assay (ELISA) with an anti-tilapia IgG secondary antibody and an anti-rabbit antibody conjugated with horseradish peroxidase (HRP). Each sample was analyzed in duplicate. Tilapia anti-β-galactosidase antibody titers were calculated as the reciprocal of the highest dilution that was two standard deviations above the average optic density of the conjugate controls. Geometric mean anti-β-galactosidase antibody titers were calculated at 0, 14, 28, 42, and 54 days post initial inoculation (Fig. 1). Analysis of variance (ANOVA) of log-transformed anti-β-galactosidase antibody titers was used to evaluate the differences in antibody titers among the three experimental groups. Unfortunately, no significant difference was found among the groups, p=0.6945. The highest ELISA antibody titer achieved was 1:6400. This occurred in all study groups, even in pre-bleeds of saline control fish. Both the saline control and empty plasmid groups produced varying β-galactosidase antibody titers from week to week. Thus, the DNA vaccination with this reporter gene system did not elicit a consistent humoral immune response in tilapia. Because some fish in all three groups had high levels of β-galactosidase antibodies prior to inoculation with the three treatments, there is a possibility that tilapia encounter the bacterial β-galactosidase from their normal flora creating circulating anti-β-galactosidase antibodies. Immunization with a different reporter gene, that is novel to tilapia, may result in more encouraging data.
| Figure 1 | 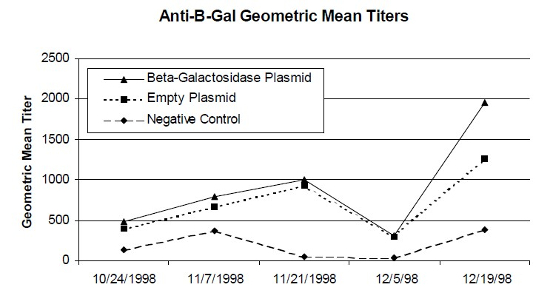
Geometric mean anti-β-galactosidase antibody titers of saline control (100 µl, IM), empty plasmid control (50 µg, IM), and test plasmid tilapia treatments (50 µg, IM). Tilapia, Oreochromis niloticus, were inoculated with treatments on 24 October 1998. |
|
| |
Literature Cited
1. Bonato VL, Lima VM, Tascon RE, Lowrie DB, Silva CL. Identification and characterization of protective T cells in hsp65 DNA vaccinated and Mycobacterium tuberculosis-infected mice. Infect Immun. 1998;66:169–175.
2. de la Fuente J, Guillen I, Martinez R, Estrada MP. Growth regulations and enhancement in tilapia: basic research findings and their applications. Genetic Analysis. 1999;15:85–90.
3. Waine GJ, Yang W, Scott JC, McManus DP, Kalinna BH. DNA-based vaccination using Schistosoma japonicum (Asian blood-fluke) genes. Vaccine. 1997;15:846–848.
4. Weinstein MR, Lilt M, Kertesz DA, Wyper P, Rose D, Coulter M, et al. 1997. Invasive infections due to fish pathogen Streptococcus iniae. S. iniae Study Group. New England J Med. 1997;337:589–594.