Abstract
Scats (feces) from Steller sea lions (Eumetopias jubatus) were collected on rookeries as a medium to compare relative levels of selected organochlorine (OC) contaminants between the thriving eastern stock in Southeast Alaska (SEA) and the depleted western stock [Gulf of Alaska (GOA) and eastern Aleutian Islands (EAI)]. Matched sets of feces, blood, and blubber samples were collected from wild pup and juvenile Steller sea lions captured during Alaska Department of Fish and Game capture operations in SEA and Prince William Sound (GOA) as well as from captive adults at the Alaska SeaLife Center. These sets were used to evaluate relationships of contaminant levels and composition in the three media. Samples were analyzed for selected congeners of polychlorinated biphenyls (PCBs) and metabolites of dichloro-diphenyl-trichloroethane (DDT) by high-performance liquid chromatography. Hair was also collected from wild-caught individuals from SEA and GOA and subsequently analyzed for total mercury (Hg). In addition, we examined the utility of porphyrin profiles in feces as a biomarker of environmental contaminant exposure in both individual sea lion feces and from rookery scats. Blood levels of individual PCBs congeners and DDT metabolites were highly correlated with blubber levels. In contrast, feces were not well correlated with blubber or blood congener profiles. This result was expected since fecal OC levels reflect excretion of PCBs congeners not metabolized or retained in the body in addition to recent dietary intake. Therefore, use of fecal OCs were intended as a rough indicator of exposure levels, not as a reflection of the individual congeners in body depot stores. We determined that OC contamination in Steller sea lions from portions of the western stock (EAI) have significantly higher OC levels excreted in feces compared to the GOA and SEA. Additionally, the mean ratio of porphyrins in scats from rookeries were correlated with OC levels. Finally, total mercury analyses indicated significantly higher levels in hair in GOA pups compared to SEA. Therefore, adverse effects of both inorganic and organic environmental contaminants need to be considered as contributing factors in the continuing decline of the western stock of SSL. This research leads us to recommend that additional, extensive, effects-based contaminant research on Steller sea lions is warranted.
Introduction
Steller sea lion (SSL) numbers in western Alaska (west of 144°W) have declined by about 80% over the past 20–30 years, and that population is now federally classified as endangered. In Southeast Alaska, sea lion numbers have nearly doubled over the same time period. While nutritional stress is the favored hypothesis for explaining the decline, other factors cannot be discounted. Organochlorine contaminants are present in the marine ecosystem of the North Pacific Ocean and relatively high concentrations have been found in the tissues of dead adult SSLs in Alaska. Several lines of evidence indicate that contaminants should be evaluated as an alternative hypothesis for explaining, in part, the decline in the western population of SSLs.
1. Levels of OCs in tissues of small samples of SSLs from Alaska have been “surprisingly” high.6,8,9,15 In addition, sea otters (Enhydra lutris) sampled from the Aleutian Islands had much higher levels of both PCBs and DDTs than otters sampled from SEA.13
2. The OC levels reported for Alaskan SSLs are high enough to be of concern, relative to levels reported to be immunosuppressive in harbor seals (Phoca vitulina).1,13
3. Several observations have been made of animals from the western population that are suggestive of immunosuppression. Dermal fungal patches have been observed more frequently on animals from the western population than in the thriving eastern population in SEA (unpublished data). Zenteno-Savin, et al.16 reported higher levels of the acute-phase protein haptoglobin from SSLs in the western population than for animals in the SEA population.
4. The ability to metabolize OC compounds appears to be less well developed in a similar species, the southern sea lion (Otaria flavescens), than in other mammals.14
5. SSLs have extended lactation and nurse their young for up to several years,10 providing an efficient mechanism for transfer of lipophilic, biomagnified contaminants vulnerable juveniles.
6. Reduced reproductive success has been reported as an adverse effect of high body burdens of OC contaminants.2,5,12 SSLs from the depressed, western Alaska population were found to have high rates of reproductive failure; about 40% of pregnancies failed, primarily the result of middle- and late-term abortions.10,11
To determine if SSLs from the two stocks are exposed to significantly different levels of OCs, we measured OC concentrations in SSL scats collected on rookeries from portions of the ranges of the western and eastern stocks. We looked for correlations between contaminant levels and composition and population trends at individual rookeries and between the Southeast Alaska and western Alaska populations. Additionally, we examined the suitability of porphyrin profiles (intermediates in the biosynthesis of heme) in feces as a biomarker of OC exposure. We compared the profiles of PCB congeners and DDT metabolites in the blood, blubber and feces of individuals to examine the relationships between the difference matrices and the suitability of using less invasive sampling techniques, as well as comparing mean levels of contaminants between regions.
Methods
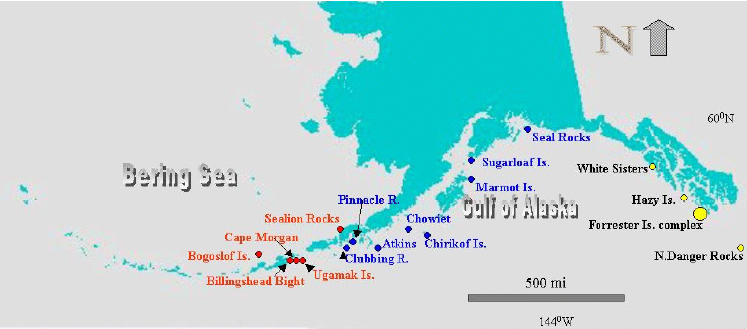
Samples of fresh feces were obtained per rectum or from observed defecation from SSL juveniles during the course of capture operations in SEA and the GOA during 1998–2000. Samples were collected with acetone-rinsed spatulas or forceps and transferred to acetone-rinsed foil or Teflon and sealed in a Whirl-Pak or into methylene chloride-rinsed vials and frozen. From these same individuals, blubber biopsies and blood samples were also obtained and frozen to −20°C until analysis. Feces as individual, recognizable defecations was collected off 18 rookeries during 1998 and 2000 (Figure 1) and handled similarly. No samples from the central or western Aleutian Islands were available. Rookery scats are primarily from adult females, as territorial bulls are fasting and pups are suckling at the season of collection. Ten to 12 scats per rookery were combined by homogenization and became a composite analyzed as a single sample from that site on that day. The same homogenate was used for both OC and porphyrin analyses.
Figure 1. Steller sea lion (SSL) rookery and haul-out scat sampling sites (1998 and 2000)
Fecal, blood, and blubber samples were extracted and analyzed for selected OC contaminants using high-performance liquid chromatography (HPLC) with photodiode array detection.7 Lipid concentrations of all samples were determined by thin-layer chromatography with flame ionization detection so comparisons of OC contaminants could be made based on lipid weight (l.w.).
Porphyrins were extracted from dried fecal samples using a hydrochloric acid extraction protocol outlined in Taylor, et al.14 Characterization of the porphyrin profiles was accomplished using high-performance liquid chromatography. Hair clipped in preparation of the blubber biopsy site was collected and prepared for total mercury determination by hot acid digestion (70% NNO3/30% H2SO4) and analyzed by SnCl2 reduction, dual gold amalgamation, and CVAFS detection (EPA method 1631 modified).
Results
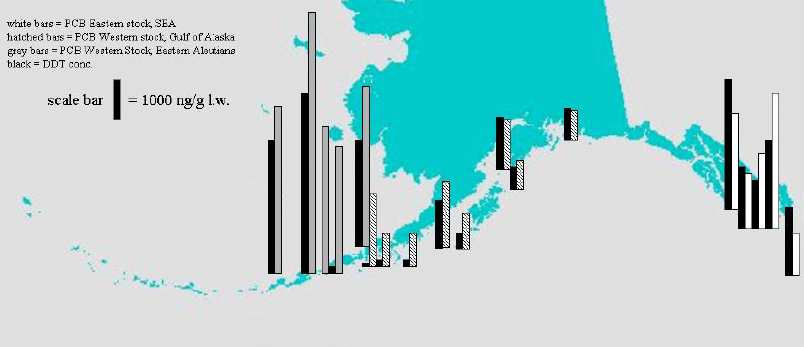
Blood levels of individual congeners of PCBs and DDT metabolites were highly correlated with blubber levels (Table 1) and therefore blood sampling is a suitable alternative to the more invasive blubber biopsy for monitoring of OC contaminants. Congener profiles in feces were not well correlated with blubber or blood congener profiles. We determined that SSLs from portions of the western stock (EAI) have significantly higher OC levels excreted in feces compared to SSL in the GOA and SEA (Figure 2). Additionally, the ratio of coproporphyrin III to uroporphyrin mean levels is higher and the mean ratio of protoporphyrin IX to coproporphyrin III is lower in the EAI and GOA rookeries than in the reference SEA rookeries. These results indicate that the animals from the western stock have a disruption at either or both the coproporphyrinogen and protoporphyrinogen oxidase steps. The porphyrin ratios were correlated to OC levels, but OC and mercury levels were inversely correlated. Therefore, increased coproIII:uro may be an appropriate biomarker of OC exposure in SSL but not mercury exposure (at least not in suckling pups). Finally, total mercury analyses indicated significantly higher levels in post-molt GOA (Prince William Sound) pups compared to SEA pups (mean±SEM, 1986±342 ng/g GOA, n=15; 876±116 SEA, n=7, p=0.43).
Table 1. Pearson correlation coefficients between selected PCB congener and
DDT concentrations in blubber vs. blood of 10 Steller sea lions, 1998–2000 (ng/g l.w.)
|
|
CB-101
|
CB-105
|
CB-138
|
CB-153
|
pp′-DDE
|
I:PCB
|
I:DDT
|
|
r
|
0.792
|
0.951
|
0.880
|
0.864
|
0.932
|
0.884
|
0.934
|
|
p
|
0.00637
|
0.00357
|
0.000778
|
0.00267
|
0.00026
|
0.000696
|
0.00008
|
CBs -118, -128, -180 not significant, CBs -110, -170 had too few above detection limits to evaluate.
Figure 2. PCBs (shaded) and DDTs (black) in SSL scats collected at rookeries or haul-outs (1998 and 2000)
Discussion
These findings indicate that PCBs are present in the food web exploited by SSL in Alaska, at least as far west as the EAI. In SEA where SPCB levels were intermediate, the population has increased in recent years. The relatively high levels for the EAI, in comparison of the nearby GOA, are of interest as they suggest either a local source or a strong influence from the Bering Sea. The animals inhabiting these areas appear to be at increased risk of adverse effects of environmental contaminant exposure.
The lack of correlation between fecal PCB congener profiles and blubber or blood was not an unexpected result. The number of animals with all three media analyzed was small. Also, fecal OC levels reflect excretion of PCB congeners not metabolized or retained in the body in addition to recent dietary intake. Thus, use of fecal OCs was intended as a rough indicator of the magnitude of exposure, not as a reflection of the individual congener concentrations in body depot stores.
Analyses of fecal composites for porphyrin metabolites, a suggestive biomarker of nonspecific contaminant exposure, indicate a “backup” of the OC metabolic pathway in the GOA and EAI samples compared to SEA samples. In light of the results of these preliminary studies, the further use of biomarkers and other studies examining adverse health effects as a result of environmental contaminant exposure are currently being undertaken by the authors with additional collaborators.
Acknowledgments and Disclaimer
We gratefully acknowledge the additional contributions of M. Krahn, A. Trites, T. Loughlin, P. Tuomi and the many Alaska Department of Fish and Game capture crewmembers as well as National Marine Mammal Laboratory personnel who assisted in the collection of samples. Although some of the research described has been funded in part by NOAA, it has not been subjected to NOAA review and therefore does not necessarily reflect the views of the NOAA, and no official endorsement should be inferred.
Literature Cited
1. De Swart, R.L., P.S. Ross, J.G. Vos, and A.D.M.E. Osterhaus. 1996. Impaired immunity in harbour seals (Phoca vitulina) exposed to bioaccumulated environmental contaminants: review of long-term feeding study. Environ. Health. Perspect. 104: 823–828.
2. DeLong, R.L., W.G. Gilmartin, and J.G. Simpson. 1973. Premature births in California sea lions: Association with high organochlorine pollutant residue levels. Science. 181: 1168–1170.
3. Estes, J.A., C.E. Bacon, W.M. Jarman, R.J. Norstrom, R.G. Anthony, and A.K. Miles. 1997. Organochlorines in sea otters and bald eagles from the Aleutian archipelago. Mar. Pollut. Bull. 34: 486.
4. Fossi, M.C., L. Marsili, M. Junin, H. Castello, J.A. Lorenzani, S. Casini, C. Savelli, and C. Leonzio. 1997. Use of nondestructive biomarkers and residue analysis to assess the health status of endangered species of pinnipeds in the southwest Atlantic. Mar. Pollut. Bull. 34: 157–162.
5. Helle, E., M. Olsson, and S. Jensen. 1976. DDT and PCB levels and reproduction in ringed seal from Bothnian Bay. Ambio. 5: 188–189.
6. Krahn, M. M. Letter and table reporting results of chlorinated hydrocarbon analyses from blubber samples of adult, female Steller sea lions from Southeast Alaska. 1997. In: Alaska Department of Fish and Game Contract Report. Steller Sea Lion Recovery Investigations in Alaska, 1995–1996. Pitcher, K.W. (ed). Pp. 71–73.
7. Krahn, M.M., G.M. Ylitalo, J. Buzitis, C.A. Sloan, D.T. Boyd, S.-L. Chan, and U. Varanasi. 1994. Screening for planar chlorobiphenyl congeners in tissues of marine biota by high-performance liquid chromatography with photodiode array detection. Chemosphere. 29: 117–139.
8. Krone, C. A. Letter and tables reporting results of contaminant analyses of tissues from Steller sea lions sampled at the Pribilof Islands, Alaska. 1997. C. Krone, NWFSC, 2725 Montlake Boulevard E, Seattle, WA 98112.
9. Lee, J.S., H. Umino, R. Tatsukawa, T.R. Loughlin, and D.G. Calkins. 1996. Persistent organochlorines in Steller sea lion (Eumetopias jubatus) from the Gulf of Alaska and the Bering Sea, 1976–1981. Mar. Pollut. Bull. 32: 535–544.
10. Pitcher, K.W. and D.G. Calkins. 1981. Reproductive biology of Steller sea lions in the Gulf of Alaska. J Mammal. 63: 599–605.
11. Pitcher, K.W., D.G. Calkins, and G.W. Pendleton. 1998. Reproductive performance of female Steller sea lions: an energetics-based reproductive strategy. Can. J. Zool. 76: 2075–2083.
12. Reijnders, P.J.H. 1986. Reproductive failure in common seals feeding on fish from polluted coastal waters. Nature (London) 324: 456–457.
13. Ross, P.S., R.L. De Swart, H.H. Timmerman, P.J.H. Reijnders, J.G. Vos, H. van Loveren, and A.D.M.E. Osterhaus. 1996. Suppression of natural killer cell activity in harbor seals (Phoca vitulina) fed Baltic Sea herring. Aquat. Toxicol. 34: 71.
14. Taylor, C., L.K. Duffy, R.T. Bowyer, and G.M. Blundell. 2000. Profiles of fecal porphyrins in river otters following the Exxon Valdez oil spill. Mar. Pollut. Bull. 40: 1132–1138.
15. Varanasi, U., J.E. Stein, W.L. Reichert, K.L. Tilbury, M.M. Krahn, and S.-L. Chan. 1992. Chlorinated and aromatic hydrocarbons in bottom sediments, fish and marine mammals in U.S. coastal waters: Laboratory and field studies of metabolism and accumulation. In: Walker, C.H. and D.R. Livingstone (eds). Persistent Pollutants in Marine Ecosystems. Pergamon Press Ltd., New York, pp. 83–115.
16. Zenteno-Savin, T., M.A. Castellini, L.D. Rea, and B.S. Fadely. 1997. Plasma haptoglobin levels in threatened Alaskan pinniped populations. J. Wildl. Dis. 33: 64–71.