The Use of Oxyglobin® in Birds: Preliminary Pharmacokinetics and Effects on Selected Blood Parameters and Tissues
Abstract
Clearly, tissue oxygenation is the most important function of the cardiovascular and respiratory systems. Because of their highly specialized respiratory system, birds are more sensitive to the effects of hypoxia than mammals. As in other species, anemia has many causes including malnutrition, infection, immune-mediated diseases, parasites, or blood loss from hemorrhage. Traditional treatment for severe anemia includes either a whole blood or concentrated red blood cell transfusion. In some patients, multiple blood transfusion may be required, which is often problematic.1-3
For years synthetic blood substitutes have been investigated. Progress in purification techniques of bovine hemoglobin made Oxyglobin® (Biopure Corporation, Cambridge, MA) available to veterinarians in 1998. This colloidal product contains 13 g/dL of polymerized bovine hemoglobin and is labeled for use in dogs as supportive treatment for any type of anemia. With Oxyglobin® tissue oxygenation is improved by (1) increasing the oxygen content of blood, (2) expanding the vascular volume, (3) allowing circulating molecules of hemoglobin in plasma to reach small vascular spaces unavailable to red cells, and (4) providing a higher potency for oxygen release to the tissues than red cells.4-8
Hemoglobin-based oxygen carriers are compatible with any blood type; therefore, the need for homologous donors and crossmatching is eliminated. Theoretically, this solution could be used in any species. The objectives of this study were (1) to determine the pharmacokinetics of a single dose of 15 ml/kg of Oxyglobin® in healthy chicken, (2) to identify Oxyglobin®-associated acute changes in hematologic parameters (CBC) and plasma chemistries, (3) to evaluate selected acute tissue and organ changes (histopathology) at 1 and 7 days postinfusion using histopathology, and (4) to determine acute morbidity/mortality that occurs acutely in experimental birds during the 7-day study.
Twenty-two healthy adult SPF White Leghorn chickens ranging in weight from 1.1 to 2.1 kg were utilized (16 experimental and six control). On the day of the study, each bird was manually restrained without sedation and a 24-ga Teflon catheter was placed intravenously (IV) in the basilary vein. The birds were randomly divided in two groups (A and B). Each bird randomly received either 15 ml/kg IV of Oxyglobin® (experimental) or Hetastarch® (Baxter Health Care, Deerfield, IL) (control) over 5 min. Blood was also collected for the pharmacokinetic analysis prior to the infusion in all birds, and at the following times: in group A, at 5, 15, 30, 60 min, 2, 4, 6, 12, 24 h, 2, 3, 4, 5, 6, 7 days postinfusion. In group B, blood was collected at 15 min, 6, 10, 14, 18, 24 h postinfusion. Blood was submitted for CBC and chemistry panel prior to the infusion, then at 1, 3 and 7 days postinfusion. The group A birds were euthanatized on day 7 and the group B birds were euthanatized on day 1 postinfusion. Complete necropsies were performed on each animal and the tissues submitted for histologic examination.
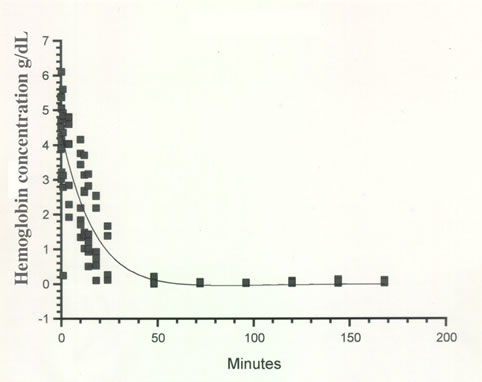
Results are summarized in Table 1. A 2-compartment pharmacokinetic model gave the best curve fit to the data (Figure 1). The distribution phase (α) had a mean half-life of 15.71 and the elimination phase (β) had a mean half-life of 15.67 h. Levels of hemoglobin reached baseline values by day 3. The elimination rate Kelim was 0.069. The value for the area under the curve (AUC) was 63.17; the transfer rate from the first compartment to the second compartment K12 was –0.0092 and from the second compartment to the first K21 was 0.028.
Table 1. Mean and standard deviation (SD) of serum concentration of hemoglobin in g/dL after a single IV slow bolus of Oxyglobin® administered at 15 ml/kg of body weight.
|
|
Time post-infusion
|
|
|
Baseline
|
Post-infusion
|
min
|
h
|
days
|
|
|
|
|
15
|
60
|
4
|
10
|
12
|
14
|
18
|
24
|
48
|
3
|
4
|
5
|
6
|
7
|
|
mean g/dL
|
0.04
|
4.29
|
4.69
|
4.41
|
3.50
|
2.51
|
2.29
|
1.74
|
1.29
|
0.83
|
0.09
|
0.03
|
0.04
|
0.04
|
0.05
|
0.04
|
|
SD
|
0.02
|
0.80
|
0.98
|
1.06
|
1.10
|
1.09
|
1.00
|
1.03
|
0.90
|
0.71
|
0.07
|
0.02
|
0.02
|
0.02
|
0.04
|
0.03
|
Figure 1. Serum concentrations of hemoglobin after a single IV slow bolus of Oxyglobin® administered at 15 ml/kg of body weight
Acknowledgments
This work was supported by the Biopure Company and grants from the Alumni Grant Funds (Cornell University) and the Raleigh-Durham Caged Bird Society.
Literature Cited
1. Degernes L.A., M.L. Crosier, L.D. Harrison, P.M. Dennis, and D.E. Diaz. 1999. Autologous, homologous, and heterologous red blood cell transfusions in cockatiels (Nymphicus hollandicus). J Avian Med Surg. 13:2–9.
2. Degernes L.A., L.D. Harrison, D.W. Smith, H.M. Newton, C.E. Ross, and D.E. Diaz. 1999. Autologous, homologous, and heterologous red blood cell transfusions in conures of the genus Aratinga. J Avian Med Surg. 13:10–14.
3. Finnegan M.V., G.B Daniel., E.C. Ramsay, 1997. Evaluation of whole blood transfusions in domestic pigeons (Columbia livia). J Avian Med Surg. 11(1):7–14.
4. Harringer W. G.T. Hodakowski, T. Svizzero, E.E. Jacobs Jr, and G.J. Vlahakes. 1992. Acute effects of massive transfusion of a bovine hemoglobin blood substitute in a canine model of hemorrhagic shock. Eur J Cardio-thorac Surg. 6:649–654.
5. Rentko, VT. 1992. Red blood cell substitute. Transfusion Medicine. 4:647–651.
6. Rentko, VT. 1996. Blood substitutes for emergency use. Proc 14th ACVIM Forum. 29–30.
7. Standl T. P. Horn, S. Wilhelm, C. Greim, M. Freitag, A. Sputtek, E. Jacobs, and J. Schulte am Esch. 1996. Laboratory investigations: bovine haemoglobin is more potent than autologous red blood cells in restoring muscular tissue oxygenation after profound isovolaemic haemodilution in dogs. Can J Anaesth. 43(7):714–723.
8. Wall R.E. 1999. Oxygen carrying fluids. Suppl Comp Cont Ed Pract Vet. 21: 2–20.