Department of Veterinary Biomedical Sciences, Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon, SK, Canada
Abstract
Birds may be more sensitive to the toxic effects of local anesthetics compared to mammals, but the pharmacokinetics of bupivacaine are unknown in birds. The purpose of this investigation was to determine the fate of bupivacaine following a single subcutaneous injection. Bupivacaine (2 mg/kg of a 0.5% solution) was given subcutaneously to eight adult female mallard ducks. Blood samples of 1.0 ml were drawn from the jugular vein prior to bupivacaine administration (0 hour) and at 0.25, 0.5, 1, 2, 3, 4, 6, and 12 hours after administration. Samples were assayed by high performance liquid chromatography and pharmacokinetic parameters were calculated from data plotted to the best-fit curve for samples from 15 to 180 min. The absorption time (T½abs=13.9 min) appeared to be shorter than the elimination time (T½elim=28.1 min) which may, in part, explain why birds are susceptible to toxic effects of local anesthetics. Maximal plasma concentration (Cmax) of 0.08±0.06 µg/ml was observed (Tmax) at 0.52±0.01 hours, but high plasma levels were evident at 6 and 12 hours (0.061 and 0.074 µg/ml, respectively). The appearance of subsequent high levels of plasma bupivacaine is unique to this study and may also contribute to the apparent avian sensitivity to toxic effects of local anesthetics.
Introduction
Local anesthetics, such as lidocaine and bupivacaine, function by blocking ion channels thereby preventing impulse conduction of pain.6 In avian species, local anesthetics are used for operative and/or post-operative pain relief.14 In domestic chickens, bupivacaine has produced effective analgesia in two pain models.9,11 Birds may be more sensitive to the toxic effects of local anesthetics compared to mammals, as higher doses (3.5–4.5 mg/kg)15 produce toxic effects in dogs compared with birds (2.7–3.3 mg/kg)11. However, pharmocokinetics of local anesthetics in avian species are unknown. The purpose of this investigation was to determine the fate of bupivacaine following a single subcutaneous injection.
Materials and Methods
Eight adult female mallard ducks, F2 progeny of wild strain adults, weighing 1060±10 g, were used for this study. Ducks were given bupivacaine (2 mg/kg, 0.4 ml/kg of a 0.5% solution, Marcaine, Sanofi Winthrop, Chatham, Ontario, Canada) subcutaneously in the ventral abdomen, between 0900 and 1100 hours. Blood samples of 1.0 ml were drawn into heparinized syringes before drug administration (0 hour) from the jugular vein and at 0.25, 0.5, 1, 2, 3, 4, 6, and 12 hours after administration. Samples were centrifuged and frozen at -20°C until analyses were performed. Concentrations of bupivacaine in plasma were determined by high-performance liquid chromatography.10
Pharmacokinetic modelling was performed using the computer program WIN-NONLIN, version 1.1 (Statistical Consultant Inc. Lexington KY, USA). Standard pharmacokinetic parameters were calculated from data plotted to the best fit curve for samples from 15 to 180 min according to a one compartment first order, first order elimination model. Area under the curve (AUC), 0 to 3 hours was computed by trapezoidal rule. All pharmacokinetic parameters were calculated for each animal and data are reported as mean ± standard deviation.
Results
One duck was excluded from the analysis as results were below detection limit of the assay from 15 to 180 min. The remaining data only fitted the model for up to 180 min. For this period the harmonic means for absorption half-life (T½abs) was 13.9 min and elimination half-life (T½elim) was 28.1 minutes. Subcutaneous administration gave an AUC (0 to 180 min) of 0.14±0.08 µg/h/L with a maximal plasma concentration (Cmax) of 0.08±0.06 µg/ml which was observed (Tmax) at 31.6 ± 6.5 minutes. High levels were also evident in five of eight ducks at 360 min (0.061±0.05 µg/ml) and in six of eight ducks at 720 min ( and 0.074±0.04 µg/ml) The plasma concentration vs. time distribution is shown in Fig 1.
Figure 1
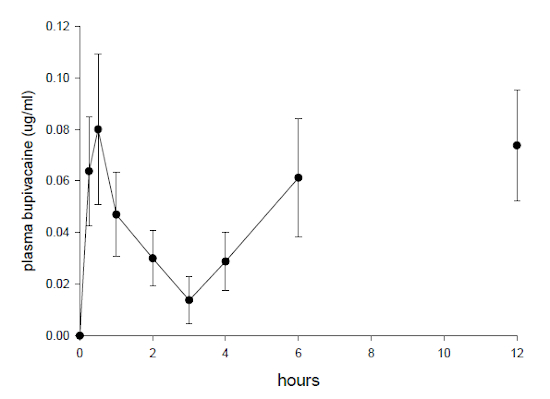
Plasma concentration of bupivacaine (µg/ml) following subcutaneous injection of 2 mg/kg of a 0.5% solution in female mallard ducks. Six and 12 hour samples represent high levels in plasma bupivacaine concentration.
Discussion
Metabolism of local anesthetics is very important because their toxicity depends largely on the balance between their rate of absorption and elimination.5 In this study the absorption rate was slower than the elimination rate, which may, in part, explain why birds are more sensitive to toxic effects of local anesthetics compared with mammals. Chickens receiving high doses of intra-articular bupivacaine (2.7–3.3 mg/kg) had evidence of toxicity (recumbency, drowsiness, and signs of distress immediately after injection).11 In dogs, plasma levels of 2.8 µg/ml are associated with increased QRS duration and conduction time,8 but plasma levels as low as 1.5–2.3 µg/ml, in humans can cause dizziness and drowsiness.7 Plasma levels required for clinical signs of toxicity in birds are unknown, but in order to avoid any serious side effects 2 mg/kg was chosen for this study based on a previous study in chickens.11 Plasma levels attained in the current study were well below levels required for toxicity in other species.
It is difficult to estimate the activity of local anesthetics from blood concentration as the amount of drug present at the site determines local anesthetic efficacy. Bupivacaine is considered to have long duration of action in comparison to other local anesthetics. When used as infiltration anesthesia, it provides approximately 2.5 to 6 hours of analgesia in dogs.15 Although it is difficult to compare studies with differing methodologies, a 15 min IV infusion of 3.4 mg/kg bupivacaine in dogs had an elimination half-life of 39.1±13.3 min.2 The length of action of bupivacaine in mallard ducks is possibly shorter as T½elim (28 min) is rapid; however, further studies are needed to confirm this.
In mammals, local anesthetics are distributed throughout all body tissues but the relative concentration in different tissues varies. Although skeletal muscle does not show any particular affinity for local anesthetics, the highest percentage of an injected dose is found in skeletal muscle because it is the largest reservoir.1 Local anesthetics are rapidly extracted by lung tissue, as extravascular pH of the lung is low relative to plasma pH and results in ion-trapping of local anesthetics.12 This first-pass lung uptake represents drug distribution not drug clearance.12 It is unknown if the avian lung is capable of similar extraction. The pH of arterial blood in chickens is 7.42–7.5213 indicating that birds are possibly more susceptible to ion trapping in the lung than mammals. However, uptake by the lung may be counteracted by greater plasma binding that occurs at higher blood pH values.1
In this study, Cmax of 0.08±0.06 was observed at 0.52±0.01 hours but high levels were also evident at 6 and 12 hours. The appearance of these high levels of plasma bupivacaine 6 and 12 hours after administration has not been recorded in other species. Pharmacokinetics of bupivacaine can depend on time of administration and, as it is highly protein bound, it may be influenced by circadian variation in protein binding.3 In birds, the lung and pectoral and gastrocnemius muscles have relatively low perfusion rates at rest but during exercise, blood flow to these tissues can increase by three to five times.4,16 It is possible that periods of activity resulting in increased blood flow through the muscle and lung, with concurrent pH changes, may produce intermittent redistribution of bupivacaine. This may also contribute to avian sensitivity to local anesthetics and suggest that delayed toxicity may be possible.
This study suggests that bupivacaine may be shorter acting in ducks than it is in mammals. A shorter absorption time, as compared to elimination time, may in part, explain avian sensitivity to local anesthetics, however, more information on drug distribution is required to draw concrete conclusions. In addition, sequestration and redistribution of bupivacaine may result in a delayed toxicity but mechanisms are unknown. Pharmacokinetics may contribute to the sensitivity of avian species to local anesthetics, but other possible mechanisms could be involved. The avian blood brain barrier is not as complex as that of mammals17 and this may allow for higher concentrations of local anesthetic in the brain. More studies are necessary to elucidate the mechanism of the toxicity of local anesthetics in avian species.
Acknowledgments
This research was supported by the Canadian Wildlife Service, the Delta Waterfowl Foundation, Ducks Unlimited’s Institute for Wetland and Waterfowl Research, and the Wildlife Health Fund, University of Saskatchewan. We also thank Donna Gauthier, for sample analysis.
Literature Cited
1. Arthur, G. R. 1987. Pharmacokinetics of local anesthetics. In: Strichartz, G. R. (ed.). Handbook of Experimental Pharmacology: Local Anesthetics. Vol 81. Springer-Verlag, Berlin. Pp. 165–186.
2. Arthur, G. R. 1988. Comparative pharmacokinetics of bupivacaine and ropivacaine, a new amide local anesthetic. Anesth. Analg. 67:1053–1058.
3. Bruguerolle, B., and M. Prat. 1987. Temporal changes in bupivacaine kinetics. J. Pharm. Pharmacol. 39: 148–149.
4. Butler, P. J. 1988. The exercise response and the ‘classical’ diving response during natural submersion in birds and mammals. Can. J. Zool. 66: 29–39.
5. Catterall, W., and K. Mackie. 1995. Local Anesthetics. In: Hardman, J. G. G., Gilman, A., and L. L. Limbird (eds). Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 9th ed. McGraw-Hill, New York, New York. Pp. 338–347.
6. Courtney, K. 1980. Structure-activity relations for frequency-dependent sodium channel block in nerve by local anesthetics. J. Pharmacol. Exp. Ther. 213: 114–119.
7. Davies, J. A., and A. J. Walford. 1986. Intravenous regional anaesthesia for foot surgery. Acta Anaesthesiol. Scand. 30: 145–147.
8. Freysz, M., Temour, Q., Mazze, R. I., Bertrix, L., Cohen, S., Samii, K., and G. Faucon. 1989. Potentiation by mild hypothermia of ventricular conduction disturbances and reentrant arrhythmias induced by bupivacaine in dogs. Anesthesiology. 70: 799–804.
9. Glatz, P.C., Murphy, L. B., and A. P. Preston. 1992. Analgesic therapy of beak-trimmed chickens. Aust. Vet. J. 69: 18.
10. Gupta, R. N., and A. Dauphin. 1994. Column liquid chromatographic determination of bupivacaine in human serum using solid-phase extraction. J. Chromatography B: Biomed. Appl. 658: 113–119.
11. Hocking, P. M., Gentle, M. J., Bernard, R., and L. N. Dunn. 1997. Evaluation of a protocol for determining the effectiveness of treatment with local analgesics for reducing experimentally induced articular pain in domestic fowl. Res. Vet. Sci. 63: 263–267.
12. Lofstrom, J. 1978. Tissue distribution of local anesthetics with special reference to the lung. Int. Anesthesiol. Clin. 16: 53–71.
13. Lukasik, V.M., E.J. Gentz, H.N. Erb, J.W. Ludders, and J.M. Scarlett. 1997. Cardiopulmonary effects of propofol anesthesia in chickens (Gallus gallus domesticus). J. Avian Med. Surg. 11(2): 93–97.
14. Ludders, J. W., and N. Mathews. 1996. Birds. In: Thurmon, J. C., Tranquilli, W.J., and G. J. Benson (eds). Lumb and Jones Veterinary Anesthesia. 3rd ed. Williams and Wilkins, Baltimore, Maryland. Pp. 645–669.
15. Skarda, R. T. 1996. Local and regional anesthetic and analgesic techniques: Dogs. In: Thurmon, J. C., Tranquilli, W. J., and G. J. Benson (eds). Lumb and Jones’ Veterinary Anesthesia. 3rd ed. Williams and Wilkins, Baltimore, Maryland. Pp. 426–447.
16. Stephenson, R. 1994. Diving energetics in lesser scaup (Aythya affinis). J. Exp. Biol. 190: 155–178.
17. Stewart, P. A., and M. J. Wiley. 1981. Structural and histochemical features of the avian blood-brain barrier. J. Comp. Neurol. 202: 157–167.