Cathy V. Williams, DVM
Abstract
Clostridium difficile is a gram-positive, anaerobic bacterium that is increasingly recognized as a serious pathogen in human and veterinary medicine. Clinical disease occurs when pathogenic strains of the organism colonize the intestinal tract, proliferate, and produce endotoxins—a process facilitated by antibiotic exposure and disruption of the normal intestinal microflora. In this report, eight cases of suspected Clostridium difficile-associated diarrhea in lemurs are described.
Introduction
Diarrhea is a common cause of morbidity and mortality in many species of captive primates, including lemurs. In two surveys of disease of lemurs in captivity, loose or abnormal stool was the most common cause for medical intervention.4,10 Reported infectious causes of enteritis in lemurs include pathogenic enteric bacteria (Salmonella typhimurium, Yersinia enterocolitica, Campylobacter fetus jejuni, enteropathic Escherichia coli, and Klebsiella pneumoniae) and gastrointestinal parasitism. Protozoal parasites, Giardia, Balantidium coli, Entamoeba, and Trichomonas are frequently associated with diarrhea in lemurs, although heavy infections with nematode parasites may be responsible as well.11 No viral enteric diseases have yet been documented in lemurs.
Clostridium difficile is an anaerobic spore-forming gram-positive rod that is recognized as the cause of enteritis in a wide variety of species. It was first identified as the etiologic agent of pseudomembranous colitis in humans in 1978.1 Clostridium difficile-associated diarrhea (CDAD) is now considered to be the most common cause of antibiotic-associated colitis in people. The spectrum of animals reported to be susceptible to developing CDAD secondary to antibiotic therapy include laboratory mammals, horses, swine, cats, penguins, ostriches, prairie dogs, marmosets, and a Kodiak bear.2,6,7,13-17,20,21 Clostridium difficile has also been implicated as causing spontaneous diarrhea not associated with antibiotic therapy in Syrian hamsters, foals, and immunocompromised humans.8,9,18
In 1998, a Coquerel’s sifaka (Propithecus verreauxi coquereli) at the Duke University Primate Center died following a protracted bout with diarrhea. Stool collected the day prior to death was positive for Clostridium difficile toxins A and B. Since then, seven more cases of suspected CDAD have occurred sporadically in lemurs at the Duke University Primate Center. The purpose of this report is to summarize these eight cases with the intent of gaining a better understanding of the clinical symptoms and factors predisposing lemurs to developing CDAD.
Methods
A retrospective survey of medical records from eight lemurs at the Duke University Primate Center exhibiting clinical symptoms of diarrhea along with positive Clostridium difficile toxin tests of stool were evaluated. Information on species, age, sex, previous antibiotic therapy, laboratory test results, and outcome of therapy were evaluated.
Routine CBC, serum chemistries, fecal Giardia (ELISA), Cryptosporidia (IFA), and C. perfringens toxin assays were sent to a commercial veterinary diagnostic laboratory (Antech Diagnostics, Farmingdale, NY). Fecal examinations for parasites and ova by direct microscopic exam and zinc sulfate flotation were performed in-house at the Duke University Primate Center, and assays for Clostridium difficile toxins A and B were performed at the Duke University Medical Center’s Clinical Microbiology Laboratory using the C. difficile Tox A/B Test (TECHLAB, Blacksburg, VA). Viral titers to primate rotavirus, parvovirus, cytomegalovirus (SA6), simian immunodeficiency virus, and simian retrovirus 1, 2, and 5 were performed on serum by Esoterix Inc. (Infectious Disease Center, San Antonio, TX).
Results
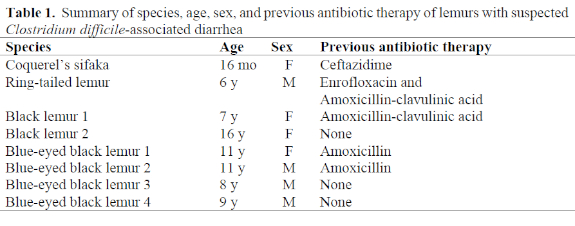
Eight cases of diarrhea associated with positive tests for Clostridium difficile toxins A and B in stools have been diagnosed in three species of lemurs at the Duke University Primate Center from June 1998 through February 2002. Species affected included one Coquerel’s sifaka (Propithecus verreauxi coquereli), one ring-tailed lemur (Lemur catta), and six black lemurs (two Eulemur macaco macaco and four Eulemur macaco flavifrons). Five of the eight lemurs received antibiotic therapy for unrelated problems prior to developing diarrhea; two lemurs were housed with animals that had developed CDAD secondary to antibiotics but had not received antibiotics themselves; and one developed diarrhea while in quarantine and did not have exposure to antibiotics nor other antibiotic-treated animals. A summary of affected animals and previous antibiotic exposure is presented in Table 1.
The severity of the symptoms and character of the diarrhea varied among individuals and among species. The Coquerel’s sifaka had profuse, watery diarrhea accompanied by complete anorexia and abdominal bloating, while the ring-tailed lemur and the black lemurs had scant, mucoid, bloody diarrhea accompanied by lesser degrees of anorexia, with or without vomiting. Complete blood counts and serum chemistries were performed on six and seven of the affected lemurs, respectively. Leukocytosis with neutrophilia was the most consistent finding present in all six animals tested. Total white blood cell counts ranged from 13,200–18,800/ml, and absolute neutrophil counts ranged from 9,850–16,360/ml. Monocytosis and lymphopenia were variably present. Biochemical abnormalities were consistent with fluid and electrolyte loss from the gastrointestinal tract and included increases in BUN and creatinine present in four of seven animals, and electrolyte abnormalities: hyperphosphatemia in five of seven, and hyponatremia and hypochloremia, each in three of seven animals. One animal each had hypoproteinemia, hypoalbuminemia, and hypoglobulinemia.
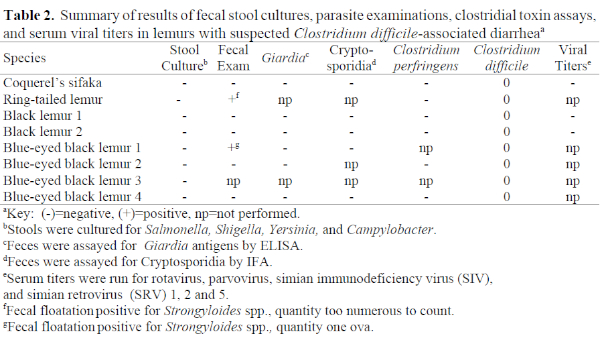
In all eight cases, testing for other known causes of diarrhea in lemurs was negative. Strongyloides spp. were found on fecal flotation exams from two animals, but treatment did not resolve the diarrhea, prompting the search for an alternate cause for the diarrhea in these animals. In addition to direct microscopic examination of feces for protozoal parasites, Giardia and Cryptosporidia were further ruled out by parasite-specific ELISA and IFA assays, respectively (see Table 2). Assays for Clostridium perfringens toxin in stool were negative in the six animals tested. Despite a scarcity of information on viral causes of diarrhea in lemurs, serum viral titers to rotavirus, parvovirus, cytomegalovirus (SA6), simian immunodeficiency virus, and simian retrovirus 1, 2, and 5 were run on three of the eight animals and were negative in all cases. Stool from the Coquerel’s sifaka was also negative for viral particles when examined by electron microscopy. In each of the eight cases, feces were positive for C. difficile toxins A and B by ELISA. Because fecal toxin detection is considered diagnostic for Clostridium difficile-associated diarrhea in other species, a tentative diagnosis of CDAD was made in each case.
Seven of the eight affected animals were treated with metronidazole at 50 mg/kg orally either q 24 h or divided BID. Clinical symptoms resolved completely in all seven animals, further supporting the diagnosis of Clostridium difficile-associated diarrhea. In the Coquerel’s sifaka, the first animal diagnosed with CDAD, the diagnosis was not made until late in the course of the illness, and the animal died 8 hours after the onset of therapy with metronidazole.
Discussion
Clostridium difficile has been isolated from a variety of sources, including marine sediment, soil and sand, and feces of clinically normal humans, mammals, and birds.3,19 While the vegetative state is strictly anaerobic, the spore form can survive in aerobic environments for many months, increasing the likelihood of environmental contamination in areas where asymptomatic carriers reside.5 Clostridium difficile is believed to cause disease after colonization of the gastrointestinal tract with toxigenic strains, proliferation of the organism, and the production of toxins. Anaerobic gut bacteria may confer resistance to colonization with C. difficile, and circumstances that alter the normal gastrointestinal tract microflora such as antibiotics may facilitate colonization. Clostridium difficile produces two potent lethal toxins, an enterotoxin (toxin A) and a cytotoxin (toxin B).12 The two toxins are believed to act synergistically to induce characteristic signs of disease. Not all strains of C. difficile possess the genes that encode for toxin production, and such strains are not considered to be clinically relevant.
Previous antibiotic therapy predisposes a variety of mammals to CDAD and this appears to be the case in lemurs evaluated in this report. Five (62%) of the lemurs described had antibiotic exposure prior to developing diarrhea. However, lemurs appear capable of developing CDAD in the absence of antibiotic therapy as well. Of the three lemurs not receiving antibiotics themselves, two were housed with animals that developed CDAD following antibiotic therapy. It is possible that heavy spore contamination of the housing environment was sufficient to cause the development of CDAD in these two animals. Heavy environmental contamination is unlikely to have caused disease in the third animal that developed CDAD while in quarantine, but stress associated with a new environment and/or a change of diet may have contributed to the development of disease in this animal.
In humans, any antibiotic can cause CDAD, but ampicillin, cephalosporins, and clindamycin are most often associated with the development of infection.5 Lemurs that developed CDAD at the Duke Primate Center following antibiotic exposure followed the same trend. One was treated with ceftazidime, a third-generation cephalosporin, and the rest were treated with amoxicillin or amoxicillin-clavulanic acid, one in combination with enrofloxacin. Similar to humans, the increased incidence of CDAD associated with the use of these antibiotics may reflect their frequent use in clinical settings rather than an inherent increased risk associated with the use of these drugs.
It is interesting that six of the eight lemurs (75%) affected with suspected CDAD were black lemurs. Whether black lemurs have an inherent increased sensitivity to the disease cannot be determined at this time. Possible alternate explanations include an increased frequency of antibiotic use in black lemurs at the Duke Primate Center relative to other species or the presence of stressors associated with the captive environment altering their resistance to C. difficile.
In summary, lemurs appear susceptible to developing spontaneous Clostridium difficile-associated diarrhea in captivity. Previous antibiotic therapy as well as proximity to other animals with the disease increases the likelihood of lemurs developing CDAD, but lemurs can develop the disease in the absence of these two factors. Diagnosis requires a high index of suspicion and depends on the clinical history, symptoms, and the demonstration of Clostridium difficile toxins A and B in the stool of affected animals. Treatment with metronidazole at 50 mg/kg divided BID is effective if started early in the course of the disease.
Acknowledgments
I would like to acknowledge Dr. Nancy Henshaw of the Duke University Medical Center for assistance in performing the Clostridium difficile toxin assays and Kelly Glenn of the Duke University Primate Center for her expert technical support.
Literature Cited
1. Bartlett, J.G., T.W. Chang, M. Gurwith, S.K. Gorbach, and A.B. Onderdonk. 1978. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N. Engl. J. Med. 298: 531–534.
2. Bartlett, J.G., T.W. Chang, N. Moon, and A.B. Onderdonk. 1978. Antibiotic-induced lethal enterocolitis in hamsters: studies with eleven agents and evidence to support the pathogenic role of toxin-producing clostridia. Am. J. Vet. Res. 39: 1525–1530.
3. Borriello, S.P., P. Honour, T. Turner, and F. Barclay. Household pets as a potential reservoir for Clostridium difficile infection. J. Clin. Pathol. 36: 84–87.
4. Brockman, D.K., M.K. Willis, and W.B. Karesh. 1988. Management and husbandry of ruffed lemurs, Varecia variegata, at the San Diego Zoo: III. Medical considerations of population management. Zoo Biol. 7: 253–262.
5. Fekety, R. 1997. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. Am. J. Gastroenterol. 92: 739–750.
6. Frazier, K.S., A.J. Herron, M.E. Hines, J.M. Gaskin, and N.H. Altman. 1993. Diagnosis of enteritis and enterotoxemia due to Clostridium difficile in captive ostriches (Struthio camelus). J. Vet. Diag. Invest. 5: 623–625.
7. Hines, R., and S. Dickerson. 1993. Pseudomembranous enteritis associated with ciprofloxacin and Clostridium difficile in a penguin (Eudyptes chrysolophus). J. Zoo Wildl. Med. 24: 553–556.
8. Jobe, B.A., A. Grasley, K.E. Deveney, C.W. Deveney, and B.C. Sheppard. 1995. Clostridium difficile colitis: an increasing hospital-acquired illness. Am. J. Surg. 169: 480–483.
9. Jones, R.L., W.S. Adney, and R.K. Shideler. 1987. Isolation of Clostridium difficile and detection of cytotoxin in the feces of diarrheic foals in the absence of antimicrobial treatment. J. Clin. Microbiol. 25: 1225–1227.
10. Junge, R.E. 1997. Medical management of the black lemur (Eulemur macaco macaco) in captivity. (Unpublished manual written for the Black Lemur SSP committee). St. Louis: St. Louis Zoo.
11. Junge, R.E. 1999. Diseases of prosimians. In: Zoo and Wild Animal Medicine, Current Therapy 4. Fowler, M.E., and R.E. Miller (eds.). W.B. Saunders Co., Philadelphia, Pp. 365–368.
12. Lyerly, D.M., H.C. Drivan, and T.D. Wilkins. 1988. Clostridium difficile: its disease and toxins. Clin. Microbiol. Rev. 1: 1–8.
13. Magdesian, K.G., J.E. Madigan, D.C. Hirsh, S.S. Jang, Y.J. Tang, T.E. Carpenter, L.M. Hansen, and J. Silva. 1997. Clostridium difficile and horses: a review. Rev. Med. Microbiol. 8 (suppl 1): S46–S48.
14. Muller, E.L., H.A. Pitt, and W.L. George. 1987. Prairie dog model for antimicrobial agent-induced Clostridium difficile diarrhea. Infect. Immun. 55: 198–200.
15. Orchard, J.L., R. Fekety, and J.R. Smith. 1983. Antibiotic-associated colitis due to Clostridium difficile in a Kodiak bear. Am. J. Vet. Res. 44: 1547–1548.
16. Rheg, J.E., and Y.S. Lu. 1981. Clostridium difficile colitis in a rabbit following antibiotic therapy for pasteurellosis. J. Am. Vet. Med. Assoc. 179: 1296–1297.
17. Rolland, R.M., L.V. Chalifoux, S.S. Snook, L.M. Ausman, and L.D. Johnson. 1997. Five spontaneous deaths associated with Clostridium difficile in a colony of cotton-top tamarins (Saguinus oedipus). Lab. Anim. Sci. 47: 472–476.
18. Ryden, E.B., N.S. Lipman, N.S. Taylor, B. Rose, and J.G. Fox. 1990. Non-antibiotic-associated Clostridium difficile enterotoxemia in Syrian hamsters. Lab. Anim. Sci. 40: 544.
19. Songer, J.G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9: 216–234.
20. Waters, E.H., J.P. Orr, E.G. Clark, et al. 1998. Typhlocolitis caused by Clostridium difficile in suckling piglets. J. Vet. Diag. Invest. 10: 104–108.
21. Weese, J.S., H.E. Weese, T.L. Bourdeau, and H.R. Staempfli. 2001. Suspected Clostridium difficile-associated diarrhea in two cats. J. Am. Vet. Med. Assoc. 218: 1436–1439.