Epizootiology and Management of Feline Leukemia Virus in Free-Ranging Florida Panthers: Preliminary Results
Abstract
Routine testing for feline leukemia virus (FeLV) antigen has been negative in Florida panthers (Puma concolor coryi) since 1983. However, between November 2002 and March 2004, four of 43 (9%) free-ranging Florida panthers tested positive for FeLV antigen. Two had a peripheral lymphadenopathy and a moderate to severe non-regenerative anemia at capture; all died within two weeks to five months of diagnosis. Antibody titers were determined for serum collected from panthers captured between 1990 and 2003; 18 of 223 (8%) were positive, with the percentage of positive titers increasing sharply beginning in 1997. Preliminary results of polymerase chain reaction (PCR) performed on archived tissues indicate virus or provirus to be present in eight panthers. Positive antigen, antibody, and PCR results were primarily from panthers sampled in the northern portion of panther range. Genetic sequencing indicates the virus to be subgroup A. Positive antibody titers and PCR results in antigen negative panthers indicate that at least some panthers do not become persistently infected. A vaccination program in free-ranging panthers was begun in August 2003, and as of March 19, 2004, 18 free-ranging panthers have been vaccinated.
Introduction
Feline leukemia virus (FeLV) is a retrovirus of domestic cats (Felis silvestris catus) that can cause anemia, neoplasia, and/or immunosuppression. Infection in wild cats is rare and has been primarily limited to case reports involving captive felids.13,15 Reports in free-ranging non-domestic cats include a mountain lion (Puma concolor) from California (U.S.)6 and a sand cat (F. margarita) from Saudi Arabia.10 Ten to 24% of European wildcats (F. sylvestris sylvestris) were also FeLV positive,2,3 although interbreeding with domestic cats occurs frequently in this subspecies.1
The Florida panther (P. concolor coryi) is a remnant population numbering approximately 80 individuals in southern Florida. This population has been the focus of intense research and genetic management since 1981. Routine testing for FeLV antigen in all captured free-ranging Florida panthers has been negative since 1983;11,12 however, the finding of two FeLV antigen positive panthers during the 2002–2003 capture season prompted an investigation and management program. The objectives of this presentation are to describe the epizootiology of FeLV infection in the Florida panther and discuss efforts to eradicate the disease.
Methods
Adult and juvenile Florida panthers and Texas cougars were captured by the Florida Fish and Wildlife Conservation Commission and National Park Service using trained hounds, chemically immobilized, and fitted with radio-collars. Neonatal kittens were handled at 1–3 weeks of age in their natal dens. Biomedical samples collected from panthers included whole blood, skin biopsies, and hair. Other samples were taken as indicated.12 Enzyme-linked immunosorbent assay (ELISA) antigen tests were performed at Cornell University (Ithaca, NY), and positive tests were confirmed by immunofluorescent antibody (IFA) at the National Veterinary Laboratory (Franklin Lakes, NJ). Immunohistochemistry of archived tissues from necropsied panthers is pending. ELISA antibody titers were determined at Hansen Veterinary Immunology (Dixon, CA). Polymerase chain reaction (PCR) and genetic sequencing were performed at the Laboratory for Viral Carcinogenesis (Frederick, MD).
To assess the safety and antibody response to vaccination, three Texas cougars and three Florida panthers underwent a vaccine trial while in captivity at White Oak Plantation. Test subjects were immobilized on three occasions at three- to four-week intervals. Two ml of Fel-O-Vax® Lv-K (Fort Dodge Animal Health, Fort Dodge, IA) were administered intramuscularly. Serum samples for ELISA antibody titers were collected at each immobilization and subjects were monitored for adverse reactions.
Results and Discussion
Since October 2002, four of 43 (9%) panthers have tested FeLV ELISA antigen positive. All positive panthers were located in Okaloacoochee Slough (OKS) in the northern portion of panther range and had overlapping home ranges. Positives represented 50% (four of eight) of panthers captured in OKS since October 2002. All were adults between two and 11 years of age and most were positive at initial capture. One panther (FP109), an 11-year-old adult, tested negative when captured in 2002 but positive when captured one year later. Two (FP122, 123) of the four antigen positives tested IFA positive, and two were inconclusive. One panther (FP115) was also feline immunodeficiency virus (FIV) positive (approximately 28% of free-ranging panthers are FIV positive).9 Coinfection with FIV in domestic cats may exacerbate the effects of FeLV infection.4
All antigen positive panthers have died. FP115 (with concurrent FIV and FeLV infections) lost 45 lb and died of an E. coli septicemia five months after capture. FP122 had a severe non-regenerative anemia (PCV 18%) and generalized lymphadenopathy at capture and died two weeks later. Other than gross signs of severe dehydration, emaciation, and anemia, necropsy results were inconclusive, and death was likely related to FeLV infection. FP109 was moderately anemic and had a generalized lymphadenopathy at capture and was killed by another male one month later. Finally, FP123, an adult male FeLV antigen/IFA positive panther, was in good condition at capture but was killed by another male two months later. This male (FP132) was captured, tested FeLV negative, and was then vaccinated. Archived tissue from eight panthers tested positive for FeLV by PCR (preliminary results), primarily in the northern portion of panther range. Positive PCR findings in some panthers may indicate latent infections. Positive findings in tissues collected at necropsy from FP109 and in experimentally aged bone marrow (sternum, FP109) indicate severely decomposed tissues may be suitable for PCR detection of FeLV. Detection of FeLV by PCR in feces collected at necropsy from FP115 indicates this technique may be useful for non-invasive monitoring for FeLV; this technique is currently being evaluated. Preliminary results of genetic sequencing indicate the virus to be subgroup A and is similar to that in domestic cats. Preliminary sequencing results also indicate that there may have been two separate introductions of the virus into the panther population. Infected domestic cats are the likely source.
ELISA antibody titers have been determined for archived serum from free-ranging panthers and Texas cougars captured between 1990 and 2003 (n=223). FP109, 115, 100, and 15 other samples were positive; the two IFA positive panthers (FP122, 123) were antibody negative. Positives were concentrated in OKS and the northern portion of panther range; positive titers decreased to the south and were non-existent south of U.S.41, including Everglades National Park. Positives have also increased dramatically since 1997 (Figure 1). Positive antibody titers were more likely among males versus females and may indicate that interactions among males (fighting) are an important mode of transmission. Positive antibody titers also appeared to increase with age.
Figure 1
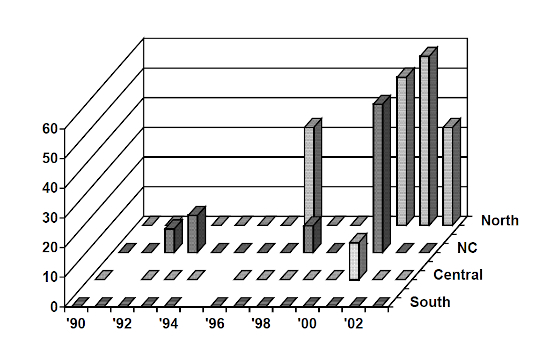
Percentage of panthers FeLV ELISA antibody positive by year and location 1990–2003. South = all areas south of U.S.41: Stair Steps Unit—Big Cypress National Preserve (BCNP), Loop Road Unit—BCNP, Everglades National Park, Southern Glades Wildlife Management Area. Central = all areas between I-75 and U.S.41. NC = public lands north of I-75. North = Okaloacoochee Slough Wildlife Management Area, Big Cypress Seminole Indian Reservation, private lands north of I-75.
Following exposure, most domestic cats clear the virus although some may remain latently infected. Reactivation of latent infections is unlikely >1 year following resolution of viremia.7 Approximately 30% of exposed domestic cats become persistently infected although susceptibility decreases with age.5 Although discordant test results can be difficult to interpret, in general, transiently infected cats will usually be antibody positive, may also be transiently antigen and PCR positive, but will remain IFA negative. Latently infected cats will have similar test results but will become PCR positive. Persistently infected cats will be positive on ELISA antigen, PCR, and IFA, although ELISA antibody titers may be negative. Preliminary antigen, PCR, and antibody results indicate that the outcome following exposure in Florida panthers is similar to that seen in domestic cats with some panthers transiently infected, some latently infected, and some persistently infected. Two of the four antigen positive panthers were also positive by IFA indicating persistent infection. Interestingly, the two IFA positive panthers were ELISA antibody negative, suggesting an inadequate immune response. The status of the two ELISA antigen positive, IFA inconclusive, panthers is unknown; however, ELISA antibody titers were positive. Transient infections may be indicated by antibody positive, but antigen and PCR negative, panthers and represent approximately 50% of panthers sampled north of I-75 over the past two years. Latent infections may be represented by antibody and PCR positive, but antigen negative, panthers.
Despite the recent introduction of Texas cougars to south Florida as part of a genetic introgression program, many panthers, especially those in the northern portion of panther range, are inbred and have a reduced genetic variation. Genetic analysis is pending, however, based on morphologic traits, we suspect that all antigen positive panthers were pure (canonical) panthers. However, antibody titers and PCRs indicate that some pure panthers and Florida panther/Texas cougar intergrades have been exposed but did not become persistently infected. Inbreeding depression and/or reduced genetic variation may have resulted in an increased susceptibility of pure panthers to FeLV. This speculation is confounded by the uneven distribution of genotypes—there is a greater percentage of pure Florida panthers in the northern portion of panther range. An examination of relatedness, genotype, and genetic variation of infected and non-infected panthers is pending.
No adverse reactions were observed in the six cougars/Florida panthers undergoing a vaccine trial in captivity. Although most subjects seroconverted, antibody titers are not necessarily indicative of vaccine efficacy in domestic cats.14 Vaccination followed by booster using Fel-O-Vax® Lv-K is safe and relatively effective in domestic cats (95–100% efficacy).14 Although a single inoculation will provide some protection, boosters are necessary to adequately protect against FeLV infection. Ideally a booster is given between three and six weeks post-inoculation; however, a booster may be sufficiently protective given between 10 days and eight weeks post-inoculation. Boosters given one year or more after a single vaccination will certainly provide at least some protection; however, controlled studies are lacking. Boosters are administered by treeing and darting the panther with the vaccine. Administering boosters is logistically difficult as well as dangerous to the panther. The decision to administer a booster depends on evidence of previous exposure, FeLV status, FIV status, location, and gender. Computer models indicate that 23–72% of domestic cats must be effectively vaccinated to achieve control in a population having a 10% prevalence of FeLV.8 As of March 19, 2004, 18 free-ranging Florida panthers have been vaccinated representing approximately 18–23% of the population.
ACKNOWLEDGMENTS
We are indebted to R. McBride and M. Roelke who are largely responsible for the success of the Florida panther recovery project. We appreciate the capture efforts of the Big Cypress National Preserve, including D. Jansen and E. Blankenship, as well as other researchers involved in the project including S. Bass, R.C. Belden, and M. Lotz. Finally, we greatly appreciate the advice and assistance of J. Levy, W. Hardy, L. Mathes, E. Hoover, J. Evermann, N. Pedersen, D. Jessup, C. Crawford, B. Ferree, and K. MacDonald. Funding for this study was provided through the Florida Panther Research and Management Trust Fund, Florida Nongame Wildlife Trust Fund, and the Federal Endangered Species Project E-1.
Literature Cited
1. Daniels MJ, Balharry D, Hirst D, Kitchener AC, Aspinall RJ. Morphological and pelage characteristics of wild living cats in Scotland: implications for defining the ‘wildcat’. J Zool Lond. 1998;244:231–247.
2. Daniels MJ, Golder MC, Jarrett O, MacDonald DW. Feline viruses in wildcats in Scotland. J Wildl Dis. 1999;35:121–124.
3. Fromont E, Sager A, Bourguemestre F, Jouquelet E, Stahl P, Pointer D, Artois M. Prevalence and pathogenicity of retroviruses in wildcats in France. Vet Rec. 2000;146:317–319.
4. Grinden CB, Corbett WT, Ammerman BE, Tomkins MT. Seroepidemiologic survey of feline immunodeficiency virus infection in cats of Wake County, North Carolina. J Am Vet Med Assoc. 1989;194:226–228.
5. Hoover EA, Olsen RG, Hardy WD Jr, Schaller JP, Mathes LE. Feline leukemia virus infection: age-related variation in response of cats to experimental infection. J Nat Cancer Inst. 1976;57:365–369.
6. Jessup DA, Pettan C, Lowenstine LJ, Pederson NC. Feline leukemia virus infection and renal spirochetosis in a free-ranging cougar (Felis concolor). J Zoo Wildl Med. 1993;24:73–79.
7. Loar AS. Feline leukemia virus immunization and prevention. Vet Clinics N Am Small Anim Pract. 1993;23:193–211.
8. Lubkin SR, Romatowski J, Zhu M, Kulesa PM, White KAJ. Evaluation of feline leukemia virus control measures. J Theoret Bio. 1996;178:53–60.
9. Olmstead RA, Langley R, Roelke ME, Goeken RM, Adger-Johnson D, Goff JP, et al. Worldwide prevalence of lentivirus infection in wild feline species: epidemiologic and phylogenetic aspects. J Virology. 1992;66:6008–6018.
10. Ostrowski S, Van Vuuren M, Lenain DM, Durand A. A serologic survey of wild felids from central west Saudi Arabia. J Wildl Dis. 2003;39:696–701.
11. Roelke ME, Forrester DJ, Jacobson ER, Kolias GV, Scott FW, Barr MC, et al. Seroprevalence of infectious disease agents in free-ranging Florida panthers (Felis concolor coryi). J Wildl Dis. 1993;29:36–49.
12. Shindle D, Cunningham M, Land D, McBride R, Lotz M, Ferree B. Florida Panther Genetic Restoration and Management. Tallahassee, FL: Florida Fish and Wildlife Conservation Commission; 2003:111.
13. Sleeman JM, Keane JM, Johnson JS, Brown RJ, Woude SV. Feline leukemia virus in a captive bobcat. J Wildl Dis. 2001;37:194–200.
14. Sparkes AH. Feline leukaemia virus and vaccination. J Feline M Surg. 2003;5:97–100.
15. Worley M. Retrovirus infections. In: Williams ES, Barker IK, eds. Infectious Diseases of Wild Mammals. Ames, IA: Iowa State University Press; 2001:213–222.