A Review of Pseudotuberculosis at a European Zoo: Epidemiology and Approaches to Control
Abstract
Epidemiologic trends of pseudotuberculosis outbreaks in callitrichids and Rodrigues’ fruit bats (Pteropus rodricensis) due to Yersinia pseudotuberculosis (Y. pstb) at Jersey Zoo are analyzed from 1981–2000. The organism appears persistent in the population with peaks of disease in winter months. Sub-adult bats (2–3 years) appear especially susceptible as do young (<2 years) and old (>9 years) callitrichids. Control of yersiniosis through vaccination and through monitoring techniques have not been effective at Jersey; however, improved husbandry methods are employed with apparent success to date.
Introduction
Yersinia pseudotuberculosis (Y. pstb) is a globally distributed facultatively anaerobic gram-negative coccobacillus. Disease may cause sudden death or chronic illness; diagnosis is most reliable at necropsy where it is characterized by necrotic and caseous lesions in liver, spleen, lung, and intestine. Almost all susceptible animals may become carriers. Disease is persistent and recurrent within populations and has zoonotic significance. Outbreaks have been reported in a wide range of species with varying susceptibility; more vulnerable species include New World primates, Indian Ocean fruit bats, and certain bird species.2
Transmission is via faecally contaminated water and food sources and ingestion of infected prey. Wild-living mammals and birds are thought to act as carriers6,7 but the relationship between the prevalence of Y. pstb in wild-living animals and infection in others is poorly understood. The bacterium may be part of the normal flora of small wild animals5. Y. pstb is reported to be widely spread in the environment in substrates such as soil, water, feces, and vegetation.4 The organism is able to survive and replicate outside of a host for years due to minimal nutritional requirements and tolerance of temperature extremes.3
Outbreaks may be precipitated by stressors such as cold and wet weather, decreases or changes in food availability, overcrowding, or capture. There is a well-established seasonal occurrence, with increased incidence associated with the colder temperatures of late autumn, winter, and early spring. The nutritional state of animals may influence their susceptibility to yersiniosis; a low-calcium environment leads to increased secretion of antiphagocytic proteins, key in the virulence process.8
Yersinia pseudotuberculosis presents a threat to captive breeding programs. The often peracute nature of the disease presents little opportunity for therapeutic intervention; it brings a potentially high mortality rate and a degree of unpredictability with likelihood of recurrence in a broad host range. Management of risk factors to limit disease is important in every collection of captive animals involved in species conservation. Methods of control available are monitoring levels of the organism, husbandry changes, and vaccination.
Methods
Raw data was obtained from postmortem reports, ARKS and MEDARKS records from 1981–2000. Deaths were attributed to Y. pstb only where the organism had been isolated and identified postmortem. The analysis was limited to previously identified susceptible groups;2 Rodrigues’ fruit bats (Pteropus rodricensis) and callitrichid species: silvery marmoset (Callithrix argentata), Geoffroy’s marmoset (Callithrix geoffroyi), cotton-top tamarin (Saguinus oedipus), golden-headed lion tamarin (Leontopithecus chrysomelas), pied tamarin (Saguinus bicolor), and Goeldi’s monkey (Calimico goeldii).
Results
From 1981–2000, a total of 28 fruit bats and 36 callitrichids were lost to yersiniosis. The pattern of losses over the years is different for these two groups, as illustrated in Figure 1. There are two main epizootics resulting in deaths in bats in 1986 (12 dead) and 1992 (seven dead), whereas the pattern of callitrichid deaths appears to be more random.
| Figure 1 | 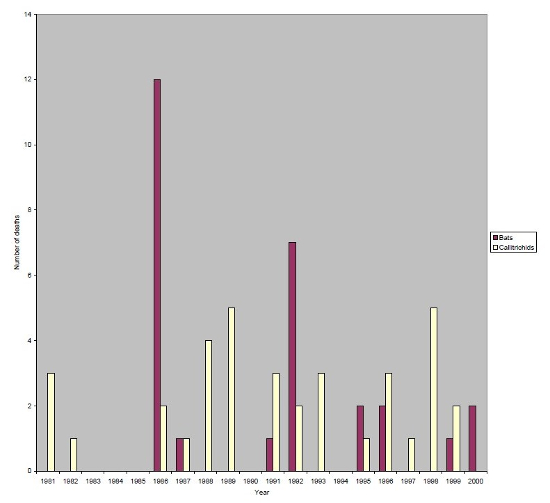
Deaths due to Yersinia pseudotuberculosis in bats and callitrichids at Jersey Zoo. |
|
| |
There is a distinct seasonal pattern in both bats and callitrichids. Two peaks, in January and March, together represent 82% of bat deaths due to yersiniosis; two peaks in March and May represent 50% of callitrichid deaths due to yersiniosis as shown in Figure 2. The summer months saw no deaths in the bats and three in the callitrichids.
| Figure 2 | 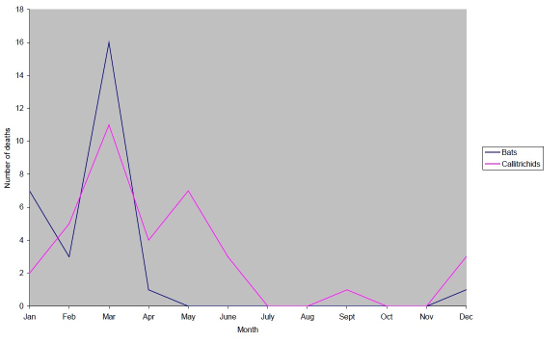
Yersinia pseudotuberculosis deaths with season (1991–2000). |
|
| |
Callitrichid species vary in susceptibility to yersiniosis, with the majority of losses in Geoffroy’s marmosets and Goeldi’s monkeys (each representing 28% of total callitrichid deaths due to yersiniosis) followed by silvery marmosets (23%). All other species each account for less than 10% of callitrichid deaths due to Y. pstb. There is no significant difference between the sexes in callitrichids lost to yersiniosis (χ2=2.314, df=1) and no apparent relationship between the enclosures in which animals are housed or the number of conspecifics in the enclosure and death due to Y. pstb. Deaths occurred in free-ranging callitrichids as well as caged. Young (≤2 years) and old (≥9 years) animals appear more susceptible than others, with a higher percentage of deaths occurring in these age groups. These two ends of the age spectrum account for 43% and 20% of deaths respectively.
There is no significant difference between the sexes in bats lost to yersiniosis (χ2=0.333, df=1) and no apparent relationship between population size and deaths due to Y. pstb. The timing of outbreaks only coincides with the maximum holding once, in 1992. The distribution of ages that succumbed to yersiniosis shown in bats is different from that in callitrichids. In bats, 60% of deaths are in those aged 2–3 years. This result does not simply reflect the age distribution within the population, as the proportion of the population of this age has not exceeded 21% since 1991. There are no deaths due to Y. pstb in bats older than 5 years.
Discussion
The total number of animals lost at Jersey is significant for a single infectious cause and could represent a serious obstacle to the success of conservation breeding programs. The pattern of incidence reflects the recurrent nature of the disease and suggests that it never completely leaves the populations, resurfacing after varying intervals; this may be due to carrier animals. Outbreaks are mostly confined to winter months; a trend particularly evident in the bats, which are kept in a hot, humid environment on a constant light cycle and should be relatively unaffected by seasonal conditions. This lends support to the idea of a wildlife reservoir as rodents may move inside in cold weather, leading to increased food contamination. Affected bats were usually in good condition; this, coupled with the age distribution, may provide an explanation in that sub-adults, although not short of food, are having to consume fallen food which is more likely to be contaminated, whether by rodents or bats, in which the disease mainly causes a necrotizing enteritis resulting in sudden death. Older bats have established perches and feeding priority. Competition for space and food could set up a chronic stress to which males and females appear equally susceptible. The lack of correlation between population size and disease in the bats is surprising, but there may be a more specific relationship between the sub-adult population and disease depending on the relative roles of competition and contamination.
The callitrichids have outside access but show a less pronounced seasonal disease pattern with a significant number of cases in late spring as well as a peak in March. This may suggest increased susceptibility among lactating or parturient females. Stress is also implicated as young and old members of the population are affected more often than others, possibly reflecting association with immune challenge. It could also reflect external stress such as lack of priority access to food and shelter due to their status in the population and subsequent increased exposure to disease risk factors. The poor condition of affected callitrichids and signs such as lethargy, heat-seeking behavior, and weakness may reflect chronic disease prior to septicemia with typical lesions on liver and spleen.
Work at Jersey has failed to demonstrate a reservoir in resident wildlife or in the soil.2 It has become established wisdom that rodents and birds act as reservoirs for Y. pstb but details of their epidemiologic role are still unclear. They are often proposed as the principal source of contamination but convincing evidence is lacking. Might there need to be a certain level of infection within the population before the organism is found in local wildlife? If so, this would imply disease is not simply passing from wildlife to zoo animals, rather that transmission occurs as a result of interplay between numerous population and environmental factors.
The multifactorial nature of yersiniosis means that it is a difficult disease to control. Use of fecal samples for monitoring is minimally invasive but the organism is not always shed into the intestinal lumen so isolation can be problematic. It requires cold-enrichment techniques and may be sufficiently sensitive to detect only clinical cases rather than carriers. The sensitivity of polymerase chain reaction tests, currently under development, may allow more effective population screening. Serologic diagnosis is complicated by the number of serotypes and limited by cross-reactions with other organisms.4 Vaccination is widely used in Europe, mainly in zoos with recurrent outbreaks of yersiniosis, and has been advocated as a primary control method.1 No challenge trials have been performed, however, and due to the sporadic nature of the disease it is difficult to assess efficacy in field trials. A study conducted at Jersey Zoo to measure antibody titers in callitrichids following vaccination showed scant response (unpublished data). Vaccination was used for a few years at Jersey Zoo but following the study, along with deaths due to Y. pstb in six vaccinated animals and two vaccine reactions it was discontinued. Other zoos consider the vaccine effective but disadvantages include the stress involved in capturing and handling the animals for injection; repeat injections are required every 6 months, along with the time and cost involved. The development of oral vaccination may eliminate some of these problems.9
Husbandry changes at Jersey aimed at tackling stress factors in yersiniosis seem to have been effective in reducing the incidence of disease. Many changes have been made to the callitrichids’ accommodation to provide as natural an environment as possible (e.g., increased foraging opportunities, densely planted larger outside areas to provide private areas, increased distance between visitors and animals).10 In addition, efforts have been made to improve cage-cleaning routines which appeared to cause unusual stress to these animals; wood shavings provide a dry indoor environment and allow spot-cleaning which is less disruptive to the occupants. In the bats’ enclosure, more extensive branching has been provided with increased availability of feeding and roost sites. Attention has also been given to nutrition; the callitrichids regularly receive probiotics. Calcium supplements are given to lactating and older callitrichids and prophylactic broad-spectrum antibiotics to those exhibiting concerning clinical signs typically associated with yersiniosis. These changes have been in place for the last 6 years; there have been no deaths due to yersiniosis in callitrichids or bats at Jersey since 2000.
Much of the complex epidemiology of Y. pstb remains unclear; consequently, control is difficult, but by committing to actions to improve the welfare and well-being of vulnerable species in captivity the likelihood of disease can be reduced. Improved monitoring of captive populations and environments would enable zoos to better protect animals against this insidious disease.
Acknowledgments
Thank you to all the staff at Jersey Zoo, where this study was undertaken as a student elective project. Special thanks to Tony Allchurch, Ann Thomasson, and Anna Feistner.
Literature Cited
1. Bielli M, Lauzi S, Pratelli A, et al. Pseudotuberculosis in marmosets, tamarins and Goeldi’s monkeys housed at a European zoo. J Zoo Wildl Med. 1999;30(4):532–536.
2. Brice S. Screening a New World monkey colony for yersinia and investigations of Y. pseudotuberculosis and soil. Dodo J Wildl Preserv Trusts. 1995;31:139–147.
3. Brubaker RR. Factors promoting acute and chronic diseases caused by yersiniae. Clin Microbiol Rev. 1991;4:309–324.
4. Carniel E, Mollaret H. Yersiniosis. Comp Immunol Microbiol Infect Dis. 1990;13(2):51–58.
5. Fukushima H, Gomyoda M, Kaneko S. Mice and moles inhabiting mountainous areas of Shimane Peninsula as sources of infection with Yersinia pseudotuberculosis. J Clin Microbiol. 1990;28(11):2448–2455.
6. Fukushima H, Gomyoda M. Intestinal carriage of Yersinia pseudotuberculosis by wild birds and mammals in Japan. Appl Environ Microbiol. 1991;57(4):1152–1155.
7. Hayashidani H, Kanzaki N, Kaneko Y, et al. Occurrence of yersiniosis and listeriosis in wild boars in Japan. J Wildl Dis. 2002;38(1):202–205.
8. Pettersson J, Nordfelth R, Dubinina E, et al. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233.
9. Thornton EA, Smith GR. Oral vaccination against pseudotuberculosis. Vaccine. 1996;14(10):977–981.
10. Wormell D, Brayshaw M. The design and redevelopment of New World primate accommodation at Jersey Zoo: A naturalistic approach. Dodo J Wildl Preserv Trusts. 2000;36:9–19.