Abstract
Diabetes mellitus is a challenging disease to manage in primates. Treatment regimens need to be developed in accordance with the management situation. Insulin injections and monitoring blood glucose values are not always feasible, and in some instances, the only options are oral therapy and monitoring urine ketone and glucose levels. Diabetes mellitus was diagnosed in a 12-year-old male L’Hoest’s guenon (Cercopithecus lhoesti) based on elevated blood glucose levels and glucosuria. Fructosamine and glycosylated hemoglobin levels were higher than that of a normal male L’Hoest’s guenon collected for comparison. Due to management limitations, insulin injections and regular blood glucose measurements were not possible, therefore, monitoring was limited to tracking body weight, and urine glucose and ketones using dipsticks (Keto-Diastix, Bayer Corporation, Elkhart, IN, USA).
This male was treated for 13 months with the oral antihyperglycemic agent metformin (Glucophage, Bristol-Myers Squibb, Princeton, NJ, USA). Treatment was initiated at a dosage of 10 mg/kg orally twice daily. The dosage was gradually increased to 20 mg/kg over an 8-month period with little response in urine glucose values. At dosages of 28–32 mg/kg, a reduction in urine glucose values as well as small decrease in fructosamine was noted (Table 1), however, medication compliance decreased with the larger volume of medication. Metformin was discontinued 13 months after treatment started due to medication refusal. A trial with glipizide (compounded formulation; Sail Drug, Hemet, CA, USA) at 0.5 mg/kg orally once daily was initiated. The dosage was gradually increased to 1.0 mg/kg over a 4-month period. No change in urine glucose was noted on glipizide and medication compliance became problematic so therapy was discontinued. Insulin levels were not routinely monitored and the lack of a better response to metformin may have been related to insufficient endogenous insulin levels.
Table 1. Blood glucose, fructosamine, and glycosylated hemoglobin values in a diabetic L’Hoest’s guenon (Cercopithecus lhoesti)
|
Date
|
Blood glucosea mmol/L (mg/dL)
|
Fructosamineb µmol/L
|
Glycosylated hemoglobinb %
|
Comments
|
|
Jul 2001
|
13.5 (244)
|
358
|
8.5
|
No therapy
|
|
Feb 2002
|
12.4 (224)
|
414
|
3c
|
No therapy
|
|
Aug 2002
|
15.0 (271)
|
422
|
10.4
|
On metformin (12.5 mg/kg)
|
|
Mar 2003
|
14.4 (260)
|
367
|
11.2
|
On metformin (32 mg/kg)
|
|
Mar 2004
|
16.8 (302)
|
427
|
14.4
|
No therapy
|
|
June 2005
|
20.6 (371)
|
400
|
11.2
|
No therapy
|
|
Jul 2006
|
25.3 (456)
|
403
|
18
|
No therapy; initial episode of rear limb paresis
|
|
Sep 2006
|
18.8 (338)
|
292
|
14.9
|
On glargine for 3 weeks
|
|
Nov 2006
|
14.2 (256)
|
305
|
14.5
|
On glargine for 9 weeks
|
aObtained under anesthesia with the guenon fasted the morning of examination; no treatments administered; performed on ExpressPlus chemistry analyzer (Bayer Corporation, Elkhart, IN, USA)
bNon-diabetic male L’Hoest’s guenon fructosamine = 196 µmol/L, glycosylated hemoglobin = 7.8%
cQuestionable result
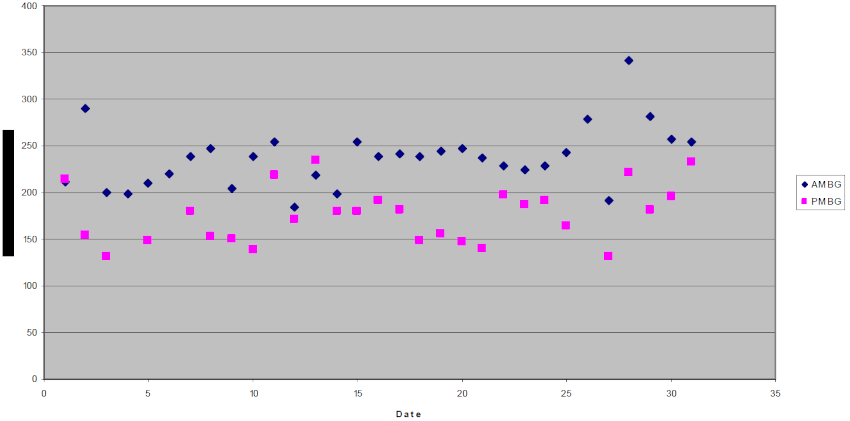
Additional therapy was not pursued until a change in exhibit housing 2.5 years later made training for insulin injections and monitoring blood glucose with a glucometer feasible. A plan to treat this guenon with insulin glargine (Lantus, Aventis Pharmaceuticals, Bridgewater, NJ, USA) was formulated. Challenges with training and changes in personnel delayed the start of treatment for 6 months. Insulin glargine at a dose of three units subcutaneously once daily was started in August 2006. Treatment was interrupted twice due to posterior paresis associated with lumbar vertebral instability requiring a dorsal hemilaminectomy. Following surgery, insulin glargine was reinitiated at the same dose. This dose was gradually increased based on blood glucose values and increasing food consumption to 5–6 units of insulin glargine once daily. Insulin glargine is a recombinant human insulin analog with glycine and arginine amino acid substitutions. It is long-acting insulin intended for once daily usage in humans. Insulin glargine does not produce a pronounced peak effect reducing the potential for hypoglycemia.1,2 Twice daily insulin injections have been used historically in diabetic primates at the San Diego Zoo. Longer-acting insulin was used in the afternoon in an attempt to compensate for the longer time between the afternoon and next morning injection, however, large fluctuations in blood glucose values were frequently seen. It was not feasible in this case to space the injections greater than 7 hours apart. Figure 1 illustrates the guenon’s morning and afternoon blood glucose values on insulin glargine during March 2007. Treatment with insulin glargine decreased the fluctuations in blood glucose and once daily injections simplified treatment. While glycosylated hemoglobin values changed little over time, the fructosamine levels dropped below values from the previous 5 years (Table 1). One disadvantage of insulin glargine is the high cost. Otherwise, insulin glargine has been an effective alternative to other insulin regimes.
Figure 1. Morning (AM) and afternoon (PM) blood glucose (BG) values during March 2007 in a diabetic L’Hoest’s guenon (Cercopithecus lhoesti) under treatment with insulin glargine
Literature Cited
1. Barnett, A.H. 2006. Insulin glargine in the treatment of type 1 and type 2 diabetes. Vasc Health Risk Manage. 2:59–67.
2. Wang, F., J.M. Carabino, and C.M. Vergara. 2003. Insulin glargine: a systematic review of a long-acting insulin analog. Clin Ther. 25:1541–77.