The body maintains a balance of acids and bases in order to constantly maintain blood pH within a narrow range, despite the continuous generation of metabolic products. In turn, this allows the body to maintain cell enzyme systems in good operation conditions, together with the proper concentration of ionized (active) forms of various electrolytes such as Ca++ and Mg++. This influences the speed of metabolic reactions and trans-membrane transportation systems (pharmacokinetics and pharmacodynamics).
In veterinary medicine, acid-base disorders are common, since they appear in frequently found conditions such as diarrhea, vomiting, renal insufficiency, dehydration, anesthesia, pneumonia, or diseases with restricted pulmonary expansion (effusion, traumatisms, hernia, tumors, etc.). Disease typically results in altered local/systemic pH, due to electrolyte/water/CO2 movements. In order to establish the appropriate therapy, electrolyte variations should be interpreted considering self physiologic basics in association with clinical findings.
Several mechanisms exist that maintain this equilibrium between acids and bases, resulting in a pH level within the reference range:
1. Extracellular mechanism. This includes several buffers that either accept or release protons (H+). Therefore, they minimize pH alterations such as plasma protein, bicarbonate (HCO3-), phosphates, etc. Its operation is immediate.
2. Intracellular mechanism. Represented by proteins, hemoglobin, organic/inorganic phosphates. It is immediate.
3. Transcellular mechanism. It is mainly produced by K+/H+ ion exchange. It is immediate.
4. Respiratory mechanism. Enhancing the retention or elimination of PCO2 (as a representative of carbonic acid, in other words, "volatile acids". Its activation is relatively immediate.
H++ HCO3-↔ H2CO3 ↔ H2O+ CO2 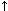 (elimination)
(elimination)
5. Renal mechanism (through the excretion or retention of H+ and HCO3-. In other words, non-volatile acids. This is the longest-lasting mechanism, since it starts in approximately 12-24 hours, and reaches its maximum compensatory efficiency peaks in 2-5 days).
NaHCO3 + HCl (a non-volatile acid) ↔ NaCl + H2CO3 ↔ H2O+ CO2
DEFINITIONS
pH. Is the negative logarithm of H+ (ratio is inverse, i.e., the higher the H+ ion concentration the lower the pH. The lower the H+ ion concentration the higher the pH). pH is determined by the PCO2 : HCO3- ratio.
pH = 6.1+ log (HCO3-/ 0.03PCO2)
Where: 6.1 is the dissociation constant of carbonic acid in body fluids, 0.03 is the CO2 solubility constant (as a conventional evaluation of H2CO3).
Acidemia. Decreased blood pH as compared to reference values.
Acidosis. A process that involves a gain of acids, a loss of bicarbonate, or both.This results in decreased pH.
Metabolic acidosis. This is the most frequent process. It is characterized by a non-volatile acid gain (mainly lactic acid) or a bicarbonate loss, or both, with an incomplete physiological respiratory compensation (PCO2 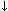 or hypocapnia). This results in decreased pH.
or hypocapnia). This results in decreased pH.
Respiratory acidosis. This process is characterized by alveolar hypoventilation that results in increased PCO2 (hypercapnia) caused by obstruction of the airways, depression of the breathing center (trauma, or drugs) breathing restrictive processes (thoracic effusion, pneumothorax, diaphragmatic hernia, abdominal distension and fractures or lesions of the thoracic walls), pulmonary disease, or a mixture of two or more causes.
Alkalemia. Increased blood pH as compared to reference values.
Alkalosis. A process characterized by a chlorine loss or decreased PCO2. This results in increased pH.
Metabolic alkalosis. A process that involves a chlorine loss or increased HCO3- (due to excess therapy), with an incomplete physiologic respiratory compensation (chemoreceptors of the breathing center detect the alkalosis and respond with hypoventilation that results in increased PCO2 [0.7 mm Hg for each mmol/L] that increases HCO3-). This results in increased pH. The most frequent causes are vomit or sequestration of chlorine in the stomach in cases of torsion. Other causes include the use of furosemide or mineralocorticoids.
Respiratory alkalosis. This is the least frequent pH disorder. It is characterized by alveolar hyperventilation that results in decreased PCO2 levels (hypocapnia) caused by hypoxemia, direct stimulation of the breathing center, stimulation of the nociceptive (pain sensitive) receptors, as in pulmonary edema, pneumonia, embolism, etc. It is accompanied by an incomplete physiological metabolic compensation (HCO3- 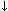 ).
).
Metabolic. A problem that results from a primary alteration in H+ or HCO3-.
Respiratory. A problem that results from a primary PCO2 change due to an alteration in CO2 elimination.
Buffers. Substances that accept or release protons (H+). They minimize pH changes such as plasma proteins, bicarbonate, phosphates, hemoglobin, etc.
PCO2 represents H2CO3 as a respiratory component of the acid-base balance.
TCO2 is the total amount ofCO2 that can be extracted from plasma. It includes dissolved CO2 and HCO3-, where H2CO3 and CO2 represent 5% of the total, while HCO3- is the remaining 95%. Therefore, TCO2 is considered as an acceptable mean of HCO3- minus 1-2 mmol/L in normal individuals.
Base excess or deficit (BE+ or BE-). This represents only the metabolic component of changes in non-volatile acids or bicarbonate, since it is estimated under ideal conditions. In other words, with a PCO2 of 40 mm Hg, a temperature of 38°C, and the buffering capacity of hemoglobin is not considered.
Reference values are typically maintained at 0 ± 3.
Values >3 = metabolic alkalosis; <- 3 = metabolic acidosis
If this value is positive, the animal does not require a bicarbonate therapy, since an excess exists. In animals with negative values, the need to correct the acid-base imbalance can be calculated using the following equation:
HCO3- dose (mmol/L)= 0.3 (treatable space) X weight in kg X BE (mmol/L)
Treatable space = extracellular fluid. Some authors consider it to be 20%.
Example: a 20 kg dog with a BE of-27 (hence a base deficit), therefore:
HCO3- dose (mmol/L) = 0.3 X 20 kg X 27
HCO3- need (mmol/L) = 162 mmol
Expected physiologic compensation. It is important to determine if the compensation is adequate or not. The following values are valid for dogs. (Cat values need to be further verified.)
 In metabolic acidosis, for each mmol/L that the HCO3- decreases, a PCO2 decrease of 0.7 mmHg is expected.
In metabolic acidosis, for each mmol/L that the HCO3- decreases, a PCO2 decrease of 0.7 mmHg is expected.
 In metabolic alkalosis, for each mmol/L that HCO3- increases a PCO2 increase of 0.7 mmHg is expected.
In metabolic alkalosis, for each mmol/L that HCO3- increases a PCO2 increase of 0.7 mmHg is expected.
 In chronic respiratory acidosis, for each mmHg that the PCO2 increases, there will be a HCO3- increase of 0.35.
In chronic respiratory acidosis, for each mmHg that the PCO2 increases, there will be a HCO3- increase of 0.35.
 In chronic respiratory alkalosis, for each mmHg that the PCO2 decreases, a HCO3- decrease of 0.55 will occur.
In chronic respiratory alkalosis, for each mmHg that the PCO2 decreases, a HCO3- decrease of 0.55 will occur.
Evaluation of blood pH/gases
The theory presented in the paragraphs above, will be explained using some examples:
SIMPLE ACID-BASE DISORDERS
|
Results |
Interpretation |
Reference |
|
pH |
7.2 |
Acidemia |
7.35-7.46 |
|
PCO2 |
62 |
Respiratory acidosis (hypercapnia) |
26-42 |
|
HCO3- |
30 |
Physiological metabolic compensation |
18-24 |
|
BE |
+12 |
Chronic process, because this compensation takes more than 12-24 hours |
(-) 3-(+) 3 |
Metabolic compensation: 62-42= 20 (PCO2); 20 X 0.35= 7; 24+7= 31
Example: pneumonia.
|
Results |
Interpretation |
Reference |
|
pH |
7.2 |
Acidemia |
7.35-7.46 |
|
PCO2 |
23 |
Physiological respiratory compensation |
26-42 |
|
HCO3- |
15 |
Metabolic acidosis |
18-24 |
|
BE |
-10 |
Base deficit due to loss or to its buffering function |
(-) 3-(+) 3 |
Example: diarrhea.
|
Results |
Interpretation |
Reference |
|
pH |
7.5 |
Alkalemia |
7.35-7.46 |
|
PCO2 |
52 |
Physiological respiratory compensation (hypercapnia) |
26-42 |
|
HCO3- |
37 |
Metabolic alkalosis |
18-24 |
|
BE |
+12 |
Base excess as a result of chlorine loss |
(-) 3-(+) 3 |
Example: vomiting.
|
Results |
Interpretation |
Reference |
|
pH |
7.5 |
Alkalemia |
7.35-7.46 |
|
PCO2 |
20 |
Respiratory alkalosis (hypocapnia) |
26-42 |
|
HCO3- |
15 |
Physiological metabolic compensation |
18-24 |
|
BE |
+12 |
Base excess |
(-) 3-(+) 3 |
Example: fear, pain, toxins, etc.
MIXED ACID-BASE DISORDERS
|
Results |
Interpretation |
Reference |
|
pH |
7.4 |
Normal |
7.35-7.46 |
|
PCO2 |
62 |
Respiratory acidosis (hypercapnia) |
26-42 |
|
HCO3- |
37 |
Metabolic alkalosis |
18-24 |
|
BE |
+12 |
Base excess as a result of chlorine loss. |
(-) 3-(+) 3 |
Expected metabolic compensation: 62-42= 20 (PCO2); 20 X 0.35= 7; 24+7= 31
Example: vomiting and pneumonia.
|
Results |
Interpretation |
Reference |
|
pH |
7.4 |
Normal |
7.35-7.46 |
|
PCO2 |
20 |
Respiratory alkalosis (hypocapnia) |
26-42 |
|
HCO3- |
12 |
Metabolic acidosis |
18-24 |
|
BE |
-12 |
Base deficit as a result of bicarbonate loss. |
(-) 3-(+) 3 |
Example: diarrhea and pain or cranial traumatism.
This interpretation is based on the following law: Compensation is never able to return pH back to reference values
|
Results |
Interpretation |
Reference |
|
pH |
7.27 |
Acidemia |
7.35-7.46 |
|
PCO2 |
39 |
Respiratory acidosis, since the expected compensation is not observed (a restrictive problem due to abdominal distension or pain) |
26-42 |
|
HCO3- |
17 |
Metabolic acidosis |
18-24 |
|
BE |
-12 |
Base deficit due to its buffering function because of acid gain |
(-) 3-(+) 3 |
Expected respiratory compensation: 18-17= 1 (HCO3); 1 X 0.7= 0.7; 26-1= 25
Example: Advanced gastric torsion, advanced pancreatitis.
A rule exists where PCO2 and HCO3- always follow the same direction, in the event of a simple problem. When direction is opposite, then it is a mixed problem, since a 20:1 ratio should exist between HCO3- and PCO2.
Electroneutrality law. A balance between cations (negative charge) and anions (positive charge) exists in the body, This electrical neutrality is always maintained. In other words, if an anion is gained, a cation is also gained. If a cation is lost, another cation is gained or an anion is lost. This maintains an always perfect balance.
ME: Cations represented by Calcium, Magnesium, Zinc, immunoglobulins, and other microelements.
NVA: non-volatile acids (both organic and inorganic acids, or anion gap), represented by lactic acid, sulfates, phosphates, ketone bodies, salts of uremic acids, and exogenous acids such as salicylates and oxalates.
Sodium and potassium represent approximately 98% of cations. Chlorine and bicarbonate represent 88% of anions. This difference represents the content of non-volatile acids (both organic and inorganic acids, or an anion gap).
The calculation of non-volatile acids (NVA) is then performed by the sum of the principal cations Na+ and K+, minus the sum of the principal anions HCO3- and Cl-.
Example: an animal with the following results:
|
Na+ : |
151 mmol/L |
|
K+ : |
5 mmol/L |
|
HCO3- |
20 mmol/L |
|
Cl- |
118 mmol/L |
Then, developing the equation:
Fixed or non-volatile acids = [(Na+) 151+ (K+) 5]-[(HCO3-) 20 + (Cl-)118]
(151+ 5)-(20+118)
156-138 = 18 mmol/L
Taking the electroneutrality law into account:
1. If a chlorine loss occur due to vomiting, gastroduodenal foreign body, torsion volvulus or ileus, this results in sequestration, and it will always exist a bicarbonate increase as renal compensation mechanism (after 12 hours). Therefore: hypochloremic metabolic alkalosis.
2. Should a bicarbonate loss occur due to diarrhea or nephropathy, increased chlorine levels will always exist. Therefore: hyperchloremic metabolic acidosis.
3. Shouldan organic acid gain occur, decreased bicarbonate levels will always result because it is used to buffer such organic acids.
Usefulness of calculating non-volatile or "fixed" acids (Anion Gap or Organic/Inorganic Acids)
1. Detecting the gain of "fixed" acids. In other words, metabolic acidosis conditions.
2. Establishing a prognosis for sick animals.
3. Using the different analytes (Na+ , K+ , HCO3-, and Cl- ) to estimate the concentration of non-volatile acids, metabolic acidosis or alkalosis processes can be determined, the problem can be located (high or low intestinal obstructions, loss of bases only, or with the generation/gain of acids, as it occurs in diarrhea with dehydration), and finally, to establish the appropriate fluid therapy.
Metabolic acidosis categories
1. Metabolic acidosis with no acid gain (non-volatile acids within the normal range). In other words, due to bicarbonate loss (diarrheas).
2. Metabolic acidosis with acid gain (high non-volatile acid levels). Examples: endogenous acidosis due to lactic acid gain (tissue hypoxia due to hypovolemia caused by profuse hemorrhage, dehydration, shock, etc.), ketone bodies, phosphates, sulfates, or in exogenous acidosis caused by the ingestion of salicylates (aspirin), oxalic acid (ethylene glycol) or methanol, among the most important ones.
Electrolyte evaluation
A routine that must be imposed in the evaluation of electrolytes is the clinical strong-ion difference (SIDc) Na+ and Cl-.
The reference values of sodium minus chlorine difference is 30 to 40 mmol/L
Example: Na+ 151 mmol/L and Cl- 118 mmol/L, then 151-118 = 33
If the difference exceeds 40, then it is a hypochloremic metabolic alkalosis (i.e., vomiting).
If the difference is lower than 30, then it is a hyperchloremic metabolic acidosis (i.e., diarrhea).
In hypochloremic metabolic alkalosis conditions, the trend is to increase blood pH (alkalemia). Therefore, the transcellular mechanism is activated in an attempt to keep pH as lease elevated as possible, through an intracellular hydrogen ion exchange with extracellular potassium ions. Therefore, in these cases we observe the trend to decreasing serum potassium levels (hypokalemia).
In metabolic acidosis conditions due to bicarbonate loss (diarrhea, low intestinal obstruction, or iliocaecal obstruction), the tendency is to decrease the pH (acidemia). Here, the transcellular mechanism is activated in an attempt to keep pH as least low as possible through an extracellular hydrogen ion exchange with intracellular potassium ions. If is a simple acid-base disorder, these cases will show increased serum potassium levels (hyperkalemia).
Summary of the most common pH/electrolyte changes associated with various clinical situations
|
|
Vomiting |
Diarrhea |
Shock |
Dehydration |
|
K+ |
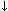
|
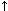
|
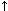
|
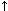
|
|
Na+ |
Normal |
Normal |
Normal |
Normal or 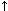 |
|
Cl- |
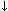
|
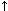
|
Normal |
Normal |
|
HCO3- |
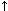
|
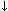
|
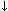
|
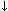
|
|
Base excess or deficit (BE) |
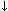
|
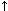
|
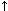
|
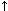
|
|
pH |
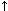
|
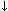
|
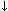
|
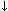
|
|
PCO2 (compensatory) |
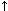
|
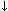
|
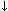
|
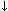
|
|
SIDC |
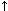
|
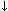
|
Normal |
Normal |
|
Non volatile acids |
Normal |
Normal |
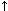
|
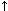
|
References
1. Adams, LG; Polzin, DJ. Mixed acid-base disorders. Vet. Clin of North Am Small An. Fluid and electrolytes disorders. 1989, 19: 2, 307-326.
2. Autran de Morais, HS. Mixed acid-base disorders. In Fluid therapy in small animal practice. DiBartola ed. W. B. Saunders, Phil. 1992: 276-296.
3. Bailey, JE; Pablo, LS. Practical aproach to acid-base disorders. Vet. Clin of North Am Small An. Advances in fluids and electrolyte disorders. 1998, 28: 3, 645-662.
4. Carlson, GP. Fluid, Electrolyte and acid-base balance. In Clinical Biochemistry of Domestic Animals. 5th Ed. Kaneko, Harvey and Bruss ed. Academic Press, San Diego Cal. 1997: 485-516.
5. DiBartola, SP. Introduction to acid-base disorders. In Fluid therapy in small animal practice. DiBartola ed. W. B. Saunders, Phil. 1992: 193-215.
6. DiBartola, SP. Metabolic acidosis. In Fluid therapy in small animal practice. DiBartola ed. W. B. Saunders, Phil. 1992: 216-243.
7. DiBartola, SP. Metabolic alcalosis. In Fluid therapy in small animal practice. DiBartola ed. W. B. Saunders, Phil. 1992: 244-257.
8. DiBartola, SP; Autran de Morais, HS. Respiratory acid-base disorders. In Fluid therapy in small animal practice. DiBartola ed. W. B. Saunders, Phil. 1992: 258-275.
9. Orsini, JA. Pathophysiology, diagnosis, and treatment of clinical acid-base disorders. Comp Cont Educ. Small An. 1989, 11: 5, 593-604.
10. Polzin, DJ; et al. Clinical application of the anion gap in evaluation of acid-base disorders. Comp Cont Educ. Small An. 1982, 4: 12, 1021-1032.
11. Robertson, SA. Simple acid-base disorders. Vet. Clin of North Am Small An. Fluid and electrolytes disorders. 1989, 19: 2, 289-306.