Associate Professor, Laboratory of Veterinary Pathology, School of Veterinary Medicine, Azabu University
Kanagawa, Japan
The existence of Helicobacter species, represented by H. pylori, has been known since the 1890s. However, it was initially believed that the bacteria could not live in an environment as acidic as pH 2, and merely passed through the digestive tract attached to food. Australian pathologist Dr. Robin Warren noted the presence of the bacillus in large numbers in the stomach of patients with gastric lesions. He and graduate student Dr. Barry Marshall succeeded in isolating H. pylori for the first time in 1982. At this time, most people did not believe in the pathogenicity of H. pylori, which was found to exist in a free state in the gastric mucin or lumen. After eradication treatment, however, the rate of recovery from gastritis and gastric ulcer is high and the rate of recurrence is low. The pathogenicity of H. pylori has gradually become clear, and it is now thought to be related in humans not only to gastritis and gastric ulcers, but also to stomach cancer and in some cases, lymphoma.
Helicobacter species chiefly inhabit the stomach. They are gram negative and (as their name implies) spiral, with strong ability to produce urease. Research has progressed not only on H. pylori, but on other Helicobacter, and over 23 types have now been classified under this group. First thought to inhabit only the stomach, they have now been found to inhabit also the intestines and bile duct system. Helicobacter are classified as either gastric or enteric, based on the region which the bacteria inhabit. Although almost all known Helicobacter have been isolated from animals, the pathogenicity of the bacteria remains poorly understood. In this lecture, I wish to introduce the types of Helicobacter found in companion animals, including their rate of infection, pathogenicity, diagnosis and treatment.
Table 1. Helicobacter species and their hosts (modified from Fox J. G., 1997)
|
Species |
Hosts |
Primary site |
Species |
Hosts |
Primary site |
|
H.pylori |
Human, monkey, cat |
Stomach |
H.bilis |
Mice, dog |
Intestine/liver, Stomach(dog) |
|
H.mustelae |
Ferret |
Stomach |
H.rodentium |
Mice |
Intestine |
|
H.felis |
Cat, dog |
Stomach |
H.trogontum |
rat |
Intestine |
|
H.bizzozeronii |
Dog, human |
Stomach |
H.muridarum |
Mice, rat |
Intestine/ Stomach |
|
H. heilmannii |
Dog, cat, human, monkey |
Stomach |
H.cinaedi |
human, hamster |
Intestine |
|
H.suis |
Pig |
Stomach |
H.fenneliae |
Human, monkey |
Intestine |
|
H.acinonyx |
Cheetah |
Stomach |
H.pullorum |
Chicken, human |
Intestine/liver (chicken) |
|
H.rappini |
Sheep, dog, human, mice |
Intestine/liver (sheep), stomach |
H.pametensis |
Chicken, pig |
Intestine |
|
H.canis |
Dog, human |
Intestine/liver (dog) |
H.cholecyctus |
Hamster |
Intestine |
|
H.hepaticus |
Mice |
Intestine/liver |
|
|
|
Helicobacter can be identified by biological characterization of bacterial culture, gene sequencing, and ultra-structural morphology. Figures show the morphology of various forms of Helicobacter from the cheetahs, as seen by electron microscopy. These include the pylori type, which is short and has a loose coiled spiral as in H. pylori; the heilmannii type, which has a long body and tightly coiled corkscrew-like spiral, and the rappini type, which has a straight cylindrical body and pericytoplasmic fibers. The heilmannii type is often observed in the stomach of animals? while in the intestines, H. cinaedi of the pylori type and H. bilis of the rappini type are often observed.
The prevalence of infection varies according to the method of observation and animal group concerned. Non-invasive methods of observation include antibody assays (serum ELISA or latex agglutination method), antigen assay (fecal ELISA method), and PCR using stool. Invasive methods include cultivation and urease examination of stomach biopsy material, histopathology (Giemsa, Warthin-Starry and immunohistological stains), and PCR using biopsy material. Among these, the most sensitive and specific test is PCR using biopsy material, followed by tissue cultivation histopathology. Prevalence of infection also varies according to the animal group concerned( e.g., between apparently healthy animals, stray animals, animals with gastrointestinal disorders and necropsied animals.
The prevalence and pathogenicity of Helicobacter infections in dogs is as follows. The rate of Helicobacter infection in dogs is reported to be as high as 67-100%. Slight gastritis can be induced by experimental inoculation, however Helicobacter infection does not induce gastric or duodenal ulcers, and no clinical signs of infection such as vomiting are seen.
The relation between Helicobacter and other gastric lesions is unknown. In our research, of 143 necropsy cases examined histopathologically (including 26 neonatal dogs which had been inoculated with canine parvo virus 1:CPV-1), Helicobacter was observed in the stomach of 23.1%. Although rate of infection in dogs 1 year old or less was slightly low at 17.9%, there was no difference in the rate of infection for dogs aged 2 years or more. Twenty-four of 103 dogs examined had chronic gastritis, but no relation between this and Helicobacter infection was identified. In the small intestine, Helicobacter was detected in 0.8% (1/114 dogs), and in the large intestine, in 33.6% (38/113 dogs). Twenty-three of 38 dogs (60.5%) found positive for Helicobacter in the large intestine were juveniles 1 year or less in age. They included many dogs experimentally infected with CPV-1 or naturally infected with CPV-2. Although colitis was seen in 12/113 dogs, no relation between this and Helicobacter infection was identified. Three dogs with inflammatory bowel disease showed no sign of Helicobacter infection.
The high prevalence of Helicobacter infection in dogs with digestive tract disease such as CPV-1 and CPV-2 infections and the fact that Helicobacter infection in such dogs extended from the large intestine and cecum to the small intestine suggested that altered environmental conditions in the digestive tract may favor proliferation of the Helicobacter. Species-wise, H. heilmannii was detected in the stomach and H. cinaedi like organisms in the intestine of infected dogs.
The prevalence of Helicobacter infection in cats reportedly ranges from 41-100% and increases with age. In histopathological examination of 111 necropsied cats (including 11 SPF cats), we found that 40.4% (38/94 cats) were infected with Helicobacter in the stomach, 1.1% (1/95) in the small intestine, and 35.2% (32/91) in the large intestine. H. heilmannii was observed in the stomach and H. bilis and loosely coiled spiral-like organisms resembling H. cineadi in the large intestine of the cats. Although gastritis and colitis were present in 57.1% (52/91) and 51.1% (48/94) of the cats respectively, no relationship was established between Helicobacter and the lesions. Helicobacter was not found in two cats which had inflammatory bowel disease. No relation was found between age, sex or breed of cat and Helicobacter infection, and Helicobacter was not detected in any organ in the SPF cats.
In the ferret, Helicobacter is reported to be present in almost all animals (100% infection rate) except the unweaned. Moreover, in contrast to the dog or cat, Helicobacter (H. mustelae) infection in ferrets causes severe gastric lesions, sometimes inducing melena and acute abdominal disease due to perforative gastric ulcer. However, in some ferrets with chronic active gastritis or perforative gastric ulcer, Helicobacter has not been found.
In the monkey, Helicobacter infection of the cotton top tamarin, associated with colitis and cancer of the colon, is well known. An H. heilmannii-like organism is also known to infect the stomach of the Macaca fascicular, a laboratory animal, in varying degrees. In Japan, many squirrel monkeys are kept in zoos, or as companion animals. We investigated Helicobacter distribution in the digestive tract of 45 squirrel monkeys from 6 facilities. Although we found no Helicobacter in the stomach, Helicobacter was identified in the large intestine and cecum of all monkeys, but no correlation with lesions such as inflammation was found. However, in the digestive tract of monkeys with digestive diseases such as Yersiniosis, the habitat of the Helicobacter extended from the large to the small intestine.
The new triple therapy for humans was found to be effective for animals as well. Experimental eradication of Helicobacter by oral administration of omeprazole 0.021 mg/head as a proton pump inhibitor combined with clarithromycin 0.24 mg/head and metronidazole 0.72 mg/head once a day for two weeks was carried out on H. heilmannii-infected mice, and successfully eradicated the bacteria. In the case of a Helicobacter-infected cheetah, based on the animal's metabolic rate, omeprazole 23.4 mg/head/day, clarithromycin 264.45 mg/head/day, and metronidazole 793.85 mg/head/day were given for two weeks. However, the rate of eradication in the cheetah was only 50%.
Click on image to see a larger view
| 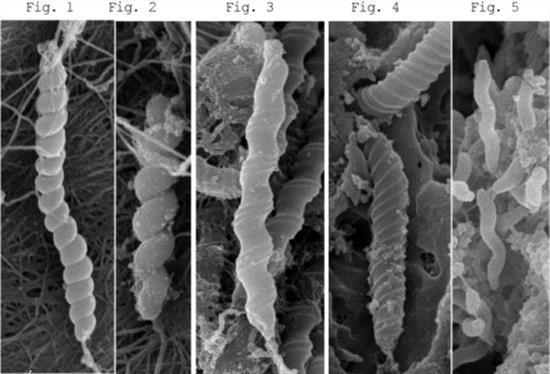
Figures 1. H.heilmannii long-type; 2. H.heilmannii short-type; 3. H.felis; 4. Flexispira rappini like organism; 5. H.acinonyx |
|
| |