Indications
 Intraosseous (IO) infusion is a recommended route for securing intravascular access in small cats and dogs, and neonates. It is particularly useful when peripheral vessels are very small or collapsed (e.g., with circulatory collapse [shock], and/or cardiac arrest). Because the vessels in the bone are supported by a rigid matrix the IO route remains useful even when all other vessels are collapsed.
Intraosseous (IO) infusion is a recommended route for securing intravascular access in small cats and dogs, and neonates. It is particularly useful when peripheral vessels are very small or collapsed (e.g., with circulatory collapse [shock], and/or cardiac arrest). Because the vessels in the bone are supported by a rigid matrix the IO route remains useful even when all other vessels are collapsed.
 The IO route can be used for the administration of resuscitation drugs (any drug that can be given intravenously), and for volume replacement with crystalloid, colloids, whole blood, or blood products.
The IO route can be used for the administration of resuscitation drugs (any drug that can be given intravenously), and for volume replacement with crystalloid, colloids, whole blood, or blood products.
 Potential access sites vary depending on the age, size and species of the animal (and include any site that is routinely used for bone marrow aspiration). In cats (and small dogs) the most frequently used route is via the proximal femur. Whichever site is used it is important that the bone selected for IO access is intact (i.e., not fractured), and that the skin over the insertion site is not damaged.
Potential access sites vary depending on the age, size and species of the animal (and include any site that is routinely used for bone marrow aspiration). In cats (and small dogs) the most frequently used route is via the proximal femur. Whichever site is used it is important that the bone selected for IO access is intact (i.e., not fractured), and that the skin over the insertion site is not damaged.
Equipment
 Clippers + skin prep solution.
Clippers + skin prep solution.
 Sterile gauze + gloves.
Sterile gauze + gloves.
 Gaseous anaesthesia + face mask, or local anaesthetic in a 1-2ml syringe + 23-25-gauge needle.
Gaseous anaesthesia + face mask, or local anaesthetic in a 1-2ml syringe + 23-25-gauge needle.
 15-18-gauge bone marrow aspiration needle (but these are too large for small neonates) or 18-22-gauge 1.5-3.0-inch spinal needle, or narrow-gauge IO infusion needle. In patients large enough to use standard bone marrow needles (e.g., Illinois or Jamshidi needles) these are usually easier to place than the specifically designed IO infusion needles (e.g., as supplied by Cook). In very small neonates 5/8-1.5-inch 18-23-gauge intravenous needles can often be used.
15-18-gauge bone marrow aspiration needle (but these are too large for small neonates) or 18-22-gauge 1.5-3.0-inch spinal needle, or narrow-gauge IO infusion needle. In patients large enough to use standard bone marrow needles (e.g., Illinois or Jamshidi needles) these are usually easier to place than the specifically designed IO infusion needles (e.g., as supplied by Cook). In very small neonates 5/8-1.5-inch 18-23-gauge intravenous needles can often be used.
 Heparinised saline flush solution.
Heparinised saline flush solution.
 T-port, intravenous fluids + tubing, suture.
T-port, intravenous fluids + tubing, suture.
Method
 Aseptically prepare the skin over the greater trochanter (hip). The point of insertion into the bone is the trochanteric fossa.
Aseptically prepare the skin over the greater trochanter (hip). The point of insertion into the bone is the trochanteric fossa.
 Depending on the stability of the patient, either 'gas down' to a light plain of anaesthesia or, if patient is to remain conscious, administer local anaesthetic to the insertion site. In the case of the latter, 1% lignocaine is injected into the subcutis over the point of insertion, then extended down to the periosteum of the trochanteric fossa. In order to avoid the sciatic nerve (which runs caudal to the femur and can be injured if the needle slips caudally from the femur) the needle should be introduced down the medial side of the greater trochanter, and moved slowly down the bone into the fossa, injecting small amounts of local anaesthetic as the needle is advanced.
Depending on the stability of the patient, either 'gas down' to a light plain of anaesthesia or, if patient is to remain conscious, administer local anaesthetic to the insertion site. In the case of the latter, 1% lignocaine is injected into the subcutis over the point of insertion, then extended down to the periosteum of the trochanteric fossa. In order to avoid the sciatic nerve (which runs caudal to the femur and can be injured if the needle slips caudally from the femur) the needle should be introduced down the medial side of the greater trochanter, and moved slowly down the bone into the fossa, injecting small amounts of local anaesthetic as the needle is advanced.
 Hold the stifle in 1 hand with your thumb laying along the long axis of the femur and the ball of the thumb over the greater trochanter. Puncture the skin with a scalpel point then direct the IO needle (whichever type has been selected) into the bone in a similar fashion to the application of the local anaesthetic (i.e., down the medial side of the greater trochanter into the trochanteric fossa) (Figure 1). Keep the needle parallel to your thumb at all times. Advance the needle with a downward "to-and-fro" rotary motion. However, very little force is needed with neonates as the bone is usually so soft that it allows the needle to pass through easily. When the needle passes through the bone into the marrow cavity, the feeling of resistance should decrease.
Hold the stifle in 1 hand with your thumb laying along the long axis of the femur and the ball of the thumb over the greater trochanter. Puncture the skin with a scalpel point then direct the IO needle (whichever type has been selected) into the bone in a similar fashion to the application of the local anaesthetic (i.e., down the medial side of the greater trochanter into the trochanteric fossa) (Figure 1). Keep the needle parallel to your thumb at all times. Advance the needle with a downward "to-and-fro" rotary motion. However, very little force is needed with neonates as the bone is usually so soft that it allows the needle to pass through easily. When the needle passes through the bone into the marrow cavity, the feeling of resistance should decrease.
| Figure 1. | 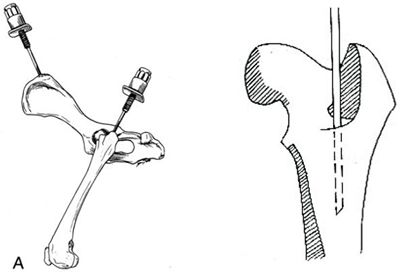
|
|
| |
Figure 1. Placement of the IO needle in the bone marrow.
A) The wing of the ileum can be useful in dogs, while the proximal femur is preferred in the cat. Picture: Slatter D, Textbook of Small Animal Surgery, third Edition, 2000, WB Saunders, Philadelphia.
B) Detail of the placement of the IO needle within the bone marrow of the proximal femur.
Picture: http://courses.vetmed.wsu.edu/samdx/bm.asp
 Once in place, the needle should be checked for proper positioning by manipulation of the femur. If an IO needle with a stylet has been used, the stylet should then be removed. In either case, the needle should be flushed with heparinised saline. If correctly positioned, saline should infuse easily into the medulla of the bone. The needle is then sutured in place, and a T-port and fluid line attached, before being covered with a protective bandage.
Once in place, the needle should be checked for proper positioning by manipulation of the femur. If an IO needle with a stylet has been used, the stylet should then be removed. In either case, the needle should be flushed with heparinised saline. If correctly positioned, saline should infuse easily into the medulla of the bone. The needle is then sutured in place, and a T-port and fluid line attached, before being covered with a protective bandage.
 Since it is not normally possible to secure the needle in place using bandage alone, it is best to suture it to the skin of the outer thigh. The leg may be restrained in place, if needed, and the infusion site should be kept clean while the needle is in place.
Since it is not normally possible to secure the needle in place using bandage alone, it is best to suture it to the skin of the outer thigh. The leg may be restrained in place, if needed, and the infusion site should be kept clean while the needle is in place.
 IO needles can be left in place for up to 72 hours, although their use is often limited by clotting within the needle. To try to reduce this risk, the needle should be flushed regularly with heparinised saline, usually every 6 hours.
IO needles can be left in place for up to 72 hours, although their use is often limited by clotting within the needle. To try to reduce this risk, the needle should be flushed regularly with heparinised saline, usually every 6 hours.
 IO fluid administration rates are limited to ~11ml/min with gravity flow and ~24ml/min with 300mmHg pressure. Care should be taken if positive pressure infusion is to be used.
IO fluid administration rates are limited to ~11ml/min with gravity flow and ~24ml/min with 300mmHg pressure. Care should be taken if positive pressure infusion is to be used.
 The following recommendations for delivering shock doses (cats 45-60ml/kg/hr; dogs up to 90ml/kg/h) come from Greg Martin (U of Queensland, Australia, see Arnold's website, below). However, care should be taken when giving fluids to small and neonatal cats and cats with potential cardiac compromise. This is because fluid overload is a very significant risk, so it is advisable to use significantly less fluid in these patients. In addition, their hydration status should be regularly assessed to ensure that over-hydration does not occur.
The following recommendations for delivering shock doses (cats 45-60ml/kg/hr; dogs up to 90ml/kg/h) come from Greg Martin (U of Queensland, Australia, see Arnold's website, below). However, care should be taken when giving fluids to small and neonatal cats and cats with potential cardiac compromise. This is because fluid overload is a very significant risk, so it is advisable to use significantly less fluid in these patients. In addition, their hydration status should be regularly assessed to ensure that over-hydration does not occur.
 Gravity flow through a single catheter can be used in animals of <7.3kg (16lb) body weight.
Gravity flow through a single catheter can be used in animals of <7.3kg (16lb) body weight.
 Pressurised flow through a single catheter or gravity flow through multiple catheters can be used in animals of 7.3-16.4kg (16 to 36lb).
Pressurised flow through a single catheter or gravity flow through multiple catheters can be used in animals of 7.3-16.4kg (16 to 36lb).
 Pressurised flow through multiple catheters can be used in animals of >16.4kg (36lb). However, a separate bone must be used for each catheter.
Pressurised flow through multiple catheters can be used in animals of >16.4kg (36lb). However, a separate bone must be used for each catheter.
 Rapid IO fluid replacement can be used to restore sufficient vascular pressure to enable routine intravenous catheter placement and continued fluid therapy.
Rapid IO fluid replacement can be used to restore sufficient vascular pressure to enable routine intravenous catheter placement and continued fluid therapy.
 Once removed, further cannulation of the same bone cannot be performed as infused fluids will leak out from the original hole into the surrounding tissue.
Once removed, further cannulation of the same bone cannot be performed as infused fluids will leak out from the original hole into the surrounding tissue.
Possible Complications
 Before attempting this procedure for the first time it is advisable to read one of the references listed below and, ideally, to practise IO needle placement on a cadaver.
Before attempting this procedure for the first time it is advisable to read one of the references listed below and, ideally, to practise IO needle placement on a cadaver.
 Occasional animals feel pain during administration of the fluids (especially if the fluids are cold or irritant, or are run in too fast).
Occasional animals feel pain during administration of the fluids (especially if the fluids are cold or irritant, or are run in too fast).
 Infection is the most common complication of IO fluid administration. It can lead to either osteomyelitis and/or subcutaneous abscess formation.
Infection is the most common complication of IO fluid administration. It can lead to either osteomyelitis and/or subcutaneous abscess formation.
 Extravasation of fluids into subcutaneous tissue may increase the risk of sterile or infected cellulitis.
Extravasation of fluids into subcutaneous tissue may increase the risk of sterile or infected cellulitis.
 Poor IO needle placement can lead to traumatic damage to the sciatic nerve.
Poor IO needle placement can lead to traumatic damage to the sciatic nerve.
 Epiphyseal injury can lead to altered bone growth.
Epiphyseal injury can lead to altered bone growth.
 Fracture of the femur is an exceedingly rare complication of this procedure.
Fracture of the femur is an exceedingly rare complication of this procedure.
 Interestingly, local haemorrhage is almost unheard of, even in cases of pre-existing thrombocytopenia and delayed clotting capability.
Interestingly, local haemorrhage is almost unheard of, even in cases of pre-existing thrombocytopenia and delayed clotting capability.
References
1. Hoskins JD (1995) Fluid therapy in the puppy and kitten. Current Veterinary Therapy XII, eds. RW Kirk and JD Bonagura, WB. Saunders, Philadelphia. pp. 34-37
2. Otto C and DT Crowe (1992), Intraosseous resuscitation techniques and applications, in Current Veterinary Therapy XI, eds. RW Kirk and JD Bonagura, Philadelphia: L&B Saunders, pp.107-109.
3. Otto, C.M., G.M. Kaufman and D.T. Crowe (1989), Intraosseous infusion of fluids and therapeutics, Compend Contin Educ Pract Vet 11 (4): 421-30
4. Greg Martin, Practical tips on intraosseous fluid administration: Arnolds Veterinary Products: www.arnolds.co.uk/intraosseousfluidtherapytips.asp
5. Washington State University, College of Veterinary Medicine, Small Animal Diagnostic & Therapeutic Techniques: Bone Marrow Aspiration: http://courses.vetmed.wsu.edu/samdx/bm.asp