Marc Vandevelde, Prof. Dr.med.vet., DECVN
Transmissible spongiform encephalopathies (TSE)
TSE are progressive lethal diseases in animals and man with very long incubation periods (years) and spongy degeneration of the CNS in the absence of inflammation and accumulation of amyloid in the nervous tissue. The amyloid is derived from a normal cellular protein, the prion protein or PrPc (c is derived from cell) which undergoes a conformational change resulting in the formation of a protease resistant isoform: PrPsc (Sc is derived from Scrapie). As a result these transformed proteins tend to polymerize into fibrils (hence the name amyloid) which are protease resistant. They cannot be recycled and accumulate in the tissue. Presumably such amyloid is toxic to the neurons. The strange thing is that, in contrast to other amyloidosis, TSE are infectious diseases. The prion theory postulates that PrPsc is capable of initiating its own replication by acting as a template for further PrPc molecules to become abnormally folded. Thus, according to the prion theory, the infectious agent consists solely of protein. Genetics of the PrP gene and experiments with transgenic mice lend strong support to the prion theory. TSE agents are very small and extremely resistant to physical and chemical treatment.
The basic neuropathology of TSE is very similar in all these conditions in humans and animals. The major differences exist in the distribution of the lesions in the CNS.
The morphological hallmark of TSE is spongiform change with formation of vacuoles in the neuropil. The second hallmark of TSE is vacuolation of neurons. In affected areas there is eventually nerve cell loss and reactive gliosis. In some of the human TSE, and to a lesser degree in some of the experimental models, protease resistant PrP occurs as plaques in the tissue. PrP Sc can be isolated and shown by electron microscopy as amyloid fibrils (scrapie associated fibrils or prion rods), or directly demonstrated with antibodies against PrP on histological sections (immunocytochemistry) or in brain homogenates (ELISA, Western blot).
TSE in humans: In humans, 4 distinct entities have been recognized: Creutzfeldt-Jakob Disease (CJD), Kuru, Gerstmann-Sträussler-Scheinker syndrome and Fatal Familial Insomnia. CJD is the most common one, albeit with only about one case worldwide per million inhabitants per year. Most cases of CJD are sporadic, about 10 % have a familial background and have been associated with numerous mutations in the PrP gene. Kuru occurred as an epidemic transmitted by cannibalistic customs in a small area in New Guinea but has nearly disappeared. The latter two are exceedingly rare familial syndromes. Variant CJD is BSE in humans and has claimed about 150 victims since 1996 mostly in GB.
Table 1. Spongiform Encephalopathies (TSEs) in different species
|
Species |
|
|
Man |
Creutzfeldt-Jakob (CJD), Kuru, variant CJD (BSE related),
Gerstmann-Stäussler-Scheinker (GSS) Syndrome,
Familial Fatal Insomnia (FFI) |
|
Sheep and Goat |
Scrapie |
|
Cattle |
Bovine Spongiform Encephalopathy (BSE, in French ESB) |
|
Mink |
Transmissible Mink Encephalopathy (TME) |
|
Zoo Ungulates |
Spongiform encephalopathy. BSE related |
|
Cat |
Feline Spongiform Encephalopathy (FSE), BSE related |
|
American Mule Deer
and Elk |
Chronic Wasting Disease (CWD) |
|
Laboratory animals |
Used for transmission experiments
(e.g., Hamster: Scrapie; Mouse: Scrapie and
BSE; Monkeys: BSE; Apes: CJD) |
Scrapie: The oldest known TSE is scrapie in sheep and goats. Scrapie in these species is a naturally occurring disease usually with a low within herd incidence. There is a clear genetic predisposition associated with polymorphisms at the level of at least three codons in the prion protein gene. In Scrapie there is replication of the infectious agent in the lymphatic tissues prior to invasion of the CNS. There is also considerable replication of the agent in the placenta which accounts for vertical transmission, probably during the perinatal period as well as horizontal transmission by contaminated pastures. Scrapie has occasionally been transmitted to farmed mink by feeding sheep offal producing transmissible mink encephalopathy.
Chronic wasting disease (CWD): This is a spontaneous TSE of deer in the US. CWD occurred as a sporadic disease in captive but also in wild living animals. Originally it was endemic in a few areas of the western United States but has recently been detected in several other parts of the country. Based on much better surveillance techniques it was recently found that the incidence of the disease was much higher than originally thought. These findings are of serious concern and programs are being developed to eradicate the disease.
Bovine spongiform encephalopathy (BSE): The most notorious member of the group is BSE with nearly 200'000 diagnosed cases since 1985. BSE was transmitted by animal protein supplements in the form of meat and bone meal (MBM). Such supplements are produced by the rendering industry and are derived from abattoir offal but also from carcasses of animals that died spontaneously. As a result of the introduction of modern continuous flow systems in the rendering industry which operate at relatively low temperatures and no longer use organic solvents to extract fat, agents of TSE became insufficiently inactivated. In Great Britain, which has a large sheep population and a high scrapie incidence, this situation had undoubtedly lead to the inclusion of active scrapie agent in the bovine diet. Once the BSE epidemic started, material derived from BSE infected cattle was also recycled for a number of years before control measures were taken, resulting in continuous enrichment of the agent in MBMs. This explains the rapid evolution of the BSE epidemic in GB and the fact that the disease got spread to other countries by export of British MBM. Export of contaminated feed and infected animals spread the disease to several other countries, giving rise to secondary--albeit small-scale epidemics.
Spread of BSE to other species
Under experimental conditions TSE are not easily transmitted from one species to another. The nature of this species barrier has not been entirely clarified but the closer the homology of the PrP gene and its product between the two involved species, the easier the transmission. By ingestion of BSE contaminated material such as certain processed meats, humans have been exposed to a considerable degree to the BSE agent especially in GB. Even though the species barrier between humans and cows appears to be high, exposure has resulted in a new form of TSE in humans, variant CJD.
Protection of the consumer consists of the confiscation of all nervous and lymphatic tissues, in which the infectious agent could theoretically be present, of all bovines (the so called Specified Bovine Risk Materials - SRM's) at the time of slaughter. This measure came into effect in the 4th year of the BSE epidemic in Great Britain and took several years to become completely effective. Rendered bovine material was also used to feed many other animal species. Fortunately, TSE has not occurred in pigs and poultry, the main species in which such feedstuffs were used. However, a small number of animals belonging to exotic ruminant and large feline species have died of BSE in zoological gardens. There are some fears that BSE may have been introduced in the sheep population. BSE has also been diagnosed in pets.
| 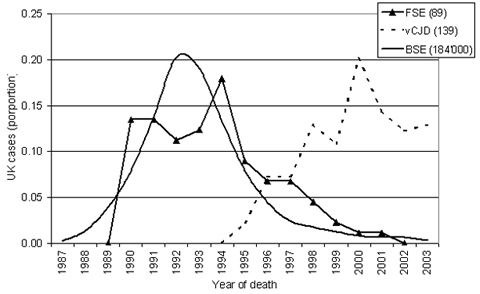
Course of BSE, Variant Creutzfeldt-Jacob disease and FSE in Great Britain. Proportional representation. (Data derived from www.defra.gov.uk, Marcus Doherr, Neurocenter, Switzerland) |
|
| |
BSE in small animals
There is no doubt that pets were exposed to the BSE agent by ingesting BSE contaminated materials, since rendered bovine material was also used in the production of commercial pet foods. Because of the enormous resistance of the BSE agent to physical and chemical inactivation procedures, high titers of infectivity remain, even in extensively processed contaminated feedstuffs. Since SRM were excluded from commercial pet foods in 1990, exposure of pets to BSE had most likely taken place before that time. An additional risk is of course the feeding of fresh offal containing CNS tissues.
Despite heavy exposure, no cases of transmissible spongiform encephalopathy have been reported in dogs since the BSE epidemic took its course. This is not because the disease could have been overlooked in this species: lesions similar to TSE have been diagnosed in dogs. A hereditary neurodegenerative condition has been reported in Rottweiler dogs which is characterized by neuronal vacuolation, spongiform change and long tract Wallerian type degeneration. This condition is not associated with accumulation of the prion protein. A few sporadic cases of extensive neuronal vacuolation of the trigeminal nuclei have been reported in dogs. However, there was no indication that this lesion was associated with TSE. While the dog appears to be resistant to BSE, a spongiform encephalopathy was discovered in cats in the course of the BSE epidemic.
Feline spongiform encephalopathy
The first cases of FSE were diagnosed in the early nineties. About a hundred cases have been reported to date, most of them in GB. One case has been described in Norway, one in Lichtenstein, two in Switzerland.
Because of the very long incubation time of spongiform encephalopathies in general, the disease affects only adult cats (peak incidence around 5 years of age). The neurological signs develop progressively over several weeks to months and are characterized by behavioural changes and gait abnormalities. There is no focalization of the neurological signs. The disease is invariably progressive and lethal. There are no typical laboratory findings. The suspicion of FSE is based on the age of the animal and the clinical signs. A variety of neurological conditions have to be considered in the differential diagnosis.
Based on strain-typing experiments, there is little doubt that FSE is derived from BSE. It is probable that cats were infected by ingesting BSE contaminated petfood. This is supported by the fact that the FSE Epidemic started to decline about five years (the average incubation period) following the ban of SRM in pet foods. Thus the peak of the epidemic in cats has certainly been passed several years ago. The number of cats being reported per year in GB has decreased dramatically. Reassuring is the fact that the vast majority of reported cases were born before 1990. Because of the spread of BSE from GB to other countries, it is to be expected that further cases of FSE will be detected for a number of years to come. However, considering the small scale of the BSE epidemics in these other countries, only few sporadic cases can be expected.
Current Risk for other species
A wide variety of tissues derived from BSE animals has been analyzed for infectivity by mouse inoculation. So far, the infectious agent has only been found in the CNS, the eye, the spinal and trigeminal ganglia. In experimental oral transmission studies in which bovines were given BSE brain orally, infectivity has also been detected in the distal ileum. The infectious agent has neither been found in muscle nor in milk. Transmission studies using intracerebral inoculation of BSE tissues directly into other bovines appear to confirm the mouse studies so far. Main protection against BSE consists of the SRM ban. Therefore, exposure to the BSE agent is extremely limited in countries with adequate TSE surveillance and in which an SRM ban exists.
Outlook
Considerable efforts have been made in recent years to improve surveillance of BSE and other TSE based on rapid tests to detect the abnormal prion protein in CNS tissues allowing the screening of large numbers of animals. Such improved surveillance techniques have detected BSE in many countries around the world, in which the disease was considered to be absent prior to 2000. This in turn has led to massive measures to prevent the spread of the disease by introducing the SRM ban and the ban on feeding animal derived materials to other animals. The latter measure originally focused on the ban of feeding animal protein to ruminants but became continuously more and more stringent. It is now prohibited to use such materials all together in farm animals. These measures are clearly showing an effect in that the BSE incidence is sharply declining in all affected countries. Because of the long incubation time, it will take several more years before the disease will be completely eradicated. The vCJD epidemic has also passed its peak. We can expect that FSE will also soon disappear. The control of Scrapie remains a challenge for the coming years.