Bernd Hoffmann, DVM, Prof. Dr. med. vet.
Klinik für Geburtshilfe, Gynäkologie und Andrologie der Groß-und Kleintiere mit Tierärztlicher Ambulanz
Gießen, Germany
INTRODUCTION
The domestic dog is a mono-oestrous, in the main aseasonal breeder with spontaneous ovulation. The reproductive phase in non-pregnant animals consists of the periods prooestrus (13-16 days), oestrus (4-12 days), dioestrus (60-90 days) and anoestrus which--depending on the breed--may vary between 15 to 265 days. In case of breeding during oestrus, dioestrus is replaced by the period of pregnancy which shows a rather constant length averaging 63 ± 2 days. It may, however, vary between 57 to 72 days due to the long period of receptivity at oestrus and the extended period of sperm survival (7 to 10 days) in the female genital tract (Tsutsui, 1998; Hoffmann et al., 1999); 70% of the follicles ovulate in the first 24 to 72 hours after the LH-peak, but ovulation at any other time during the end of pro-oestrus and oestrus is possible (Wildt et al., 1979). Following ovulation oocytes need another 2 to 3 day maturation period as a prerequisite for conception. Embryos reach the uterine horns in the blastocyst stage 10 to 12 days after onset of oestrus, implantation occurs around day 16 following the phase of inner migration (spacing).
This complex system of ovulation, fertilisation, early embryonic development and implantation is under strict hormonal control with progesterone being one of the main endocrine factors.
Corpus luteum (Cl) function and maintenance of pregnancy
As a peculiarity in the dog follicular progesterone secretion precedes ovulation, breeding and conception occur during the early phase of Cl-function with progesterone between 19 to >38nmol/l. Maximum concentrations are reached between days 10 to 20 after conception; thereafter they show a continuous decrease which turns over into a precipitous drop prior to parturition (Fig. 1). By blocking intracellular Ca++ and reducing the number of gap junctions progesterone, which is solely of luteal origin, is the key hormone to guarantee uterine quiescence during pregnancy. In addition progesterone is the main factor responsible for cervical closure. As during dioestrus in non-pregnant dogs, Cl-function in pregnant animals is independent of gonadotropic support during the first trimester of luteal life span, thereafter LH and in particular prolactin gain importance as luteotropic factors. This switch in a functional role is accompanied by an increased availability of prolactin and LH (Hoffmann et al., 1999).
Oestrogen concentrations decrease from relatively high levels during pro-oestrus to low levels during oestrus, showing no further changes during pregnancy. They decrease to basal levels concomitant with progesterone immediately prior to parturition. Apart from estradiol and estrone no other oestrogens could be detected during pregnancy in the dog, also when examining placental tissues. Hence, different to other domestic animals, there is no pregnancy specific oestrogen production in the dog and the Cl must be considered as the source of the low levels oestrogens determined (Hoffmann et al., 1994).
Course of relaxin concentrations
In the dog the polypeptide relaxin, a member of the insulin-family, is the only pregnancy specific hormone. It becomes detectable during the 4th week of pregnancy, maximum levels (4-6 ng/ml) are reached 2 to 3 weeks before parturition. They decline somewhat before whelping and remain between 0.5-2.0 ng/ml for another 4 to 9 weeks. The placenta has been considered as the main source of relaxin but also the uterus and the ovaries seem to contribute as is indicated by the still elevated levels after parturition (Steinetz et al., 1987, Hoffmann et al., 1999, Johnston et al., 2001). Until now no specific function could be attributed to the role of relaxin in the dog; apart from the well-established effect on the symphysis ossis pubis in other animal species a complimentary role to progesterone in inhibiting myometrial function has been discussed (Challis, Olsen 1988).
| Figure 1. | 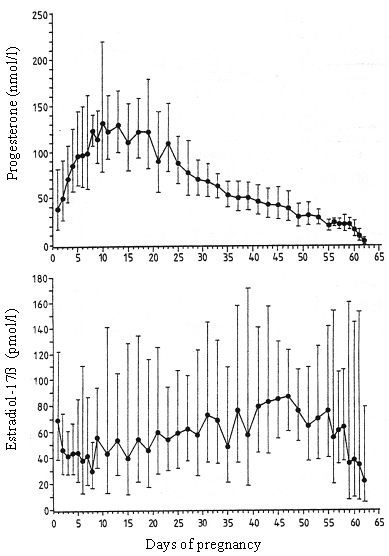
Course of progesterone and estradiol-17b concentrations during pregnancy in the dog (Hoffmann et al., 1994). |
|
| |
Hormonal changes around parturition
In non-pregnant animals cessation of luteal function starts during or at the end of the first third of dioestrus; it is a continuous, slowly ongoing process with the reaching of basal levels < 3.8 nmol/liter (1 ng/ml) at the end of dioestrus. Also in pregnant dogs the course of progesterone concentration exhibits a similar pattern; however, different to non-pregnant dogs this gradual luteal regression turns into a well-timed, precipitous progesterone decrease in pregnant animals, signaling the onset of parturition (Figure 1). Luteal regression is accomplished within a period of 16.8 ± 3.4 hours and precedes the first visible signs of onset of parturition (restlessness, nesting behaviour, rejection of food intake) which became obvious 33.6 ± 12.6 hours after onset of luteal regression. Luteolysis is accompanied by an increase in peripheral PGF2a concentration which approach maximum concentrations concomitant with the first visible signs of the onset of parturition. According to own observations the first puppy was born between 1.5 and 13.5 hours later (mean 9.6 ± 4.7 hours). Onset of labour depends on the availability of PGF2a (Challis, Olsen 1988), the final expulsion of the puppies is due to the release of oxytocin which occurs as a result of cervical stimulation shortly after PGF2a -maximum levels have been reached (Nohr, 1993; Hoffmann et al., 1999). The still present enigma relates to the mechanisms leading to luteolysis. PGF2a has shown to be luteolytic in the dog, however, only in high dosages leading to side effects or after giving repeated injections over a longer period of time. These observations point to a pharmacological rather than physiological activity. This goes along with the observation that the blocking of the prepartal PGF2a increase by Indomethacin only led to an extension of pregnancy when high dosages, also inducing side effects, were given. From these observations the hypothesis was delineated, that local, paracrine, regulatory factors, including PGF2a, might trigger luteolysis. However, recent data from our laboratory have shown that cyclooxygenase is not specifically expressed in the Cl of pregnant animals during that phase of luteolysis. The high expression observed in the placenta defines this organ as a source of the prepartal PGF2a -release. Thus the pending questions are still open.
Other hormonal changes observed in peripheral maternal plasma are a short but distinct increase of cortisol and growth hormone at the time of parturition, a possibly stress-related observation also made in other species (Hoffmann et al., 1994).
Induction of abortion and parturition
Depending on the stage of pregnancy all treatments leading to a withdrawal of the activity of progesterone result in either fetal death, followed by resorption and/or abortion or induction of parturition. This can be achieved by blocking the release of the gonadotropic hormone prolactin during the second half of pregnancy by application of dopamine agonists (e.g., Cabergoline). Similarly the use of PGF2a in a dosage leading to luteolysis induced abortions (Jonston et al., 2001). A more recent approach is the use of antiprogestins blocking the activity of progesterone at the receptor level. Fieni et al. (1996) reported about the successful use of the antiprogestin Aglepristone® for induction of abortion from the time of mating up to the last trimester of pregnancy. In our own hands application of this antiprogestin in combination with PGF2a and / or oxytocin has shown to be a successful approach for induction of parturition; no side effects were observed and the course of parturition was considered as physiological (Riesenbeck et al., 1999).
References
1. Fiéni F, Tainturier D, Bruyas JF, Badinnand F, Berthelot X, Ronsin P, Rachail M, Lefay MP (1996).: Etude clinique d'une anti-hormone pour provoquer l'avortement chez la chienne: l'aglépristone. Rec. Méd. Vét. 172, 359-367.
2. Johnston SD, Root Kustritz M, Olson PNS (2001): Canine and Feline Theriogenology. W.B. Saunders Company.
3. Nohr B (1993): Untersuchungen zur endokrinen Kontrolle der Geburt bei der Hündin unter Anwendung eines Antigestagens. Diss. med. vet., Gießen.
4. Challis FRG, Olsen DM (1988): Parturition. In: The Physiology of Reproduction, Vol. 2, Raven Press ltd., New York, 2177-2216.
5. Hoffmann B, Höveler R, Nohr B, Hasan SH (1994): Investigations on hormonal changes around parturition in the dog and the occurrence of pregnancy-specific non conjugated oestrogens. Exp. Clin. Endocrinol. 102, 185-189.
6. Hoffmann B, Riesenbeck A, Schams D, Steinetz BG (1999): Aspects on Hormonal Control of Normal and Induced Parturition in the Dog. Reprod. Dom. Anim. 34, 219-226.
7. Riesenbeck A, Klein R, Hoffmann B, Hospes R (1999): Geburtsinduktion infolge verlängerter Gravidität bei einer Hündin unter Verwendung eines Antigestagens. Tierärztl. Prax. 27 (K), 186-188.
8. Steinetz BG, Goldsmith L, Lust G (1987): Plasma relaxin levels in pregnant and lactating dogs. Biol. Re-prod. 37, 719-725.
9. Tsuitsui T (1989): Gamete physiology and timing of ovulation and fertilization in dogs. J. Reprod. Fertil. 39, 269-275.
10. Wildt DE, Panko WB, Chakraborty PK, Seager SW (1979): Relationship of serum estrone, estradiol-17b and progesterone to LH, sexual behaviour and time of ovulation in the bitch. Biol. Reprod. 20, 648-658.